Table 1.
Preparation of optically active homopropargylic allyl alcohols via CBS reduction.
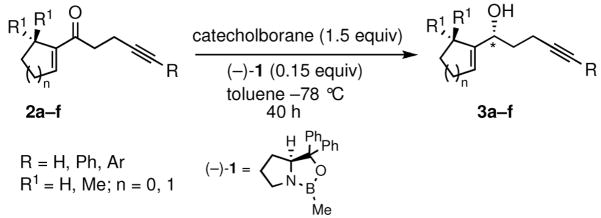 | ||||
|---|---|---|---|---|
| entry | enone (2) | allyl alcohol (3) | yield (%)a | ee (%)b |
| 1 |
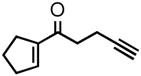 2a |
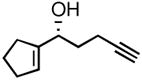 3a |
86 | 87 |
| 2 |
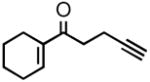 2b |
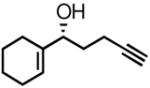 3b |
66 | 89 |
| 3 |
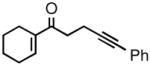 2c |
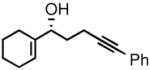 3c |
95 | 91 |
| 4 |
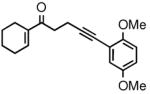 2d |
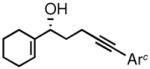 3d |
81 | 91 |
| 5 |
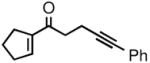 2e |
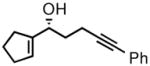 3e |
52 | 89 |
| 6 |
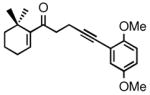 2f |
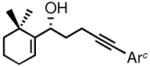 3f |
68d | 98 |
Yields of chromatographically purified products.
Enantiomer ratios were determined either by chiral GC or chiral HPLC.
Ar = 2,6-dimethoxyphenyl.
Reaction was quenched after 72 h.
