Table 2.
Synthesis of optically active bicyclic cycloheptenone derivatives via tandem 5-exocyclization/Claisen rearrangement.
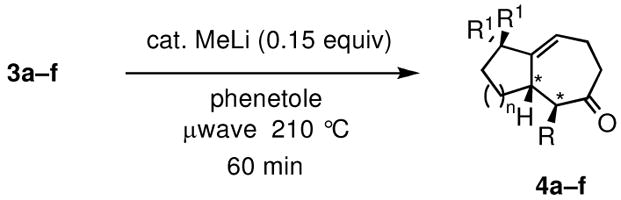 | |||||
|---|---|---|---|---|---|
| entry | allyl alcohol (3) | bicyclic ketone (4) | yield (%)a | dr | ee (%)b |
| 1 | 3a |
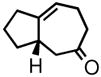 4a |
62 | 85 | |
| 2 | 3b |
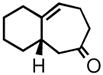 4b |
77 | 87 | |
| 3 | 3c |
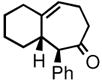 4c |
78 | 92:8 | 85 |
| 4 | 3d |
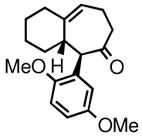 4d |
82 | 77:23 | 89 |
| 5 | 3e |
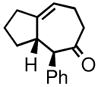 4e |
76 | 92:8 | 84 |
| 6 | 3f |
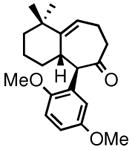 4f |
79 | 83:17(91:9)c | 95 |
Combined yield of both diastereomers.
Enantiomeric excess of the major diastereomer was determined either by chiral GC or chiral HPLC.
After 24 h of stirring in MeONa/MeOH at 25 °C.
