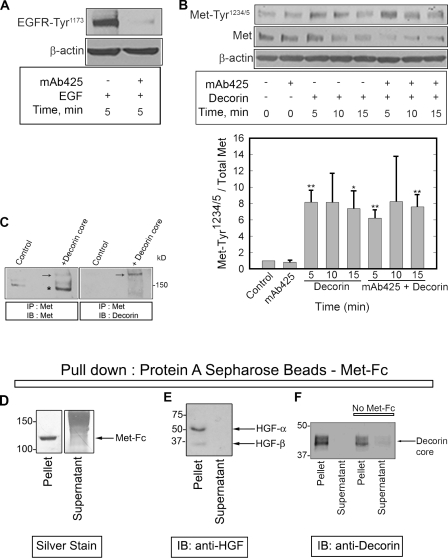
Figure 2.
Decorin interacts with the Met receptor. (A) 1 µg/ml mAb425, an EGFR-specific blocking antibody, was tested before experiments in combination with decorin by evaluating its effect in inhibiting EGF-dependent (16 nM) EGFR phosphorylation. (B, top) Immunoblot of a short decorin (100 nM) time course showing phosphorylation of the Met receptor at Tyr1234/5, total Met, and β-actin in the presence or absence of 1 µg/ml mAb425. (bottom) Quantification of immunoblots similar to those shown in the top panel. Values represent the mean ± SEM from three independent experiments performed in triplicate (*, P < 0.05; **, P < 0.01). (C) Immunoblots detecting Met (left) and decorin (right) in cells treated with decorin protein core for 15 min, cross-linked with 500 nM S-SMPB for 20 min at 37°C, and immunoprecipitated with an anti–C terminus Met antibody. Arrows point to a high Mr complex of Met and decorin protein core (∼190 kD). The asterisk indicates Met monomer (∼140 kD). (D) Silver-stained gel. Notice that the entire Met-Fc is bound to the protein A–Sepharose beads. Smear is the carrier proteins. (E) Immunoblotting (IB) of HGF after pull-down with protein A beads–Met-Fc. (F) Immunoblotting of decorin after pull-down with either protein A beads–Met-Fc or beads alone. Note the absence of HGF or decorin in the supernatants, indicating that essentially all of the ligands were bound. IP, immunoprecipitation. (D–F) Values shown are given in kiloDaltons.