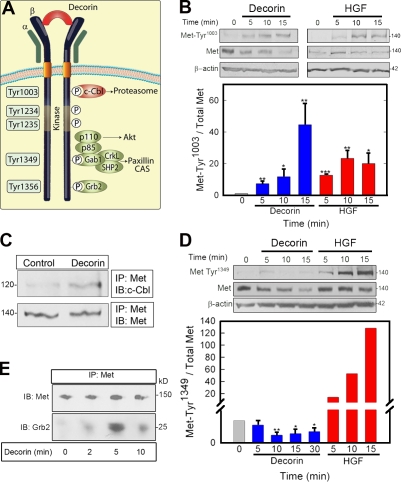
Figure 4.
Decorin induces differential and selective phosphorylation of Met Tyr residues. (A) Diagram of the main Met receptor Tyr phosphorylation sites and adaptor proteins. CAS, Crk-associated substrate; P, phosphate. (B, top) Representative immunoblots of a short decorin (100 nM) time course showing phosphorylation of the Met receptor at Tyr1003 and total Met amount vis à vis 1.5 nM HGF. (bottom) Quantification of immunoblots from three independent experiments. (C) Coimmunoprecipitation of c-Cbl and Met using an antibody directed toward the intracellular domain of Met. 100 nM decorin treatment was performed for 10 min. (D, top) Representative immunoblot showing phosphorylation of the Met receptor at Tyr1349 and total Met after 100 nM decorin treatment vis à vis 1.5 nM HGF. (bottom) Quantification of immunoblots from three independent experiments performed in triplicate. (E) Recruitment of Grb2 to the Met receptor mediated by 100 nM decorin. Coimmunoprecipitation of the Met receptor and Grb2 using an anti-Met C terminus antibody for the immunoprecipitation (IP) and either the same antibody or an anti-Grb2 monoclonal antibody for the immunoblotting (IB). Values represent the mean ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001). All of the relative values were obtained by scanning densitometry (chemiluminescence). (B–E) Values shown are given in kiloDaltons.