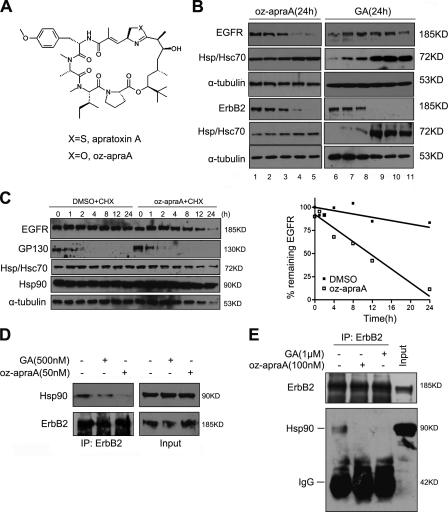
Figure 1.
Apratoxin A and its analogue inhibit the Hsp90 pathway. (A) Chemical structure of the compounds. (B) Oz-apraA reduces the levels of Hsp90 client proteins in different cell lines. Oz-apraA and GA induce concentration-dependent decreases of EGFR levels in A549 cells, ErbB2 levels in MDA-MB-453 cells at 24 h, and increases in Hsp70 levels. Lanes 1–5: 0, 1, 10, 100, and 500 nM; lanes 6–11: 0, 1, 10, 100, 500, and 1,000 nM. (C) Half-lives of EGFR and short-lived protein GP130 were analyzed in HeLa cells treated with 100 µg/ml CHX in the presence of DMSO or 100 nM oz-apraA for the indicated periods of time. The levels of EGFR were normalized to that of α-tubulin expression, and results were plotted against inhibitor treatment time points. (D) Immunoprecipitation (IP) of endogenous ErbB2-containing protein complexes from MDA-MB-453 cells after treatment of 500 nM GA or 50 nM oz-apraA for 6 h. (E) In vitro Hsp90-binding assay. SKoV3 cell lysates were incubated with 1 µM GA or 100 nM oz-apraA at 4°C for 2 h. ErbB2 was immunoprecipitated, and bound Hsp90 was detected using Western blotting.