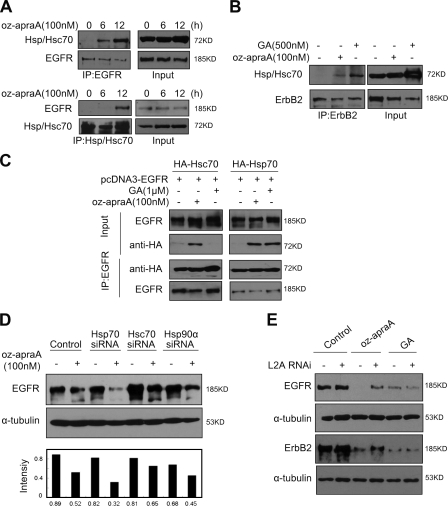
Figure 6.
Oz-apraA induces Hsp90 client membrane protein degradation through CMA. (A) Interaction of EGFR and Hsp/Hsc70 after treatment with oz-apraA. (B) Oz-apraA and GA both increase the interaction of ErbB2 and Hsp/Hsc70. (C) Oz-apraA but not GA induces the interaction between Hsc70 and EGFR. HEK293 cells were transfected with 2 µg pcDNA3-EGFR together with 2 µg pcDNA-HA-Hsc70 or pcDNA-HA-Hsp70 for 24 h followed by treatment with 100 nM oz-apraA or 1 µM GA for an additional 12 h. The cells were lysed, and the lysates were immunoprecipitated with anti-EGFR antibody followed by immunoblotting with anti-HA antibody to detect the interaction of EGFR with Hsc70 or Hsp70. (D) Hsc70 RNAi could partially inhibit oz-apraA–induced EGFR degradation. HeLa cells were transfected with siRNA specifically against Hsc70, Hsp70, or Hsp90 for 48 h followed by treatment with or without 100 nM oz-apraA for an additional 12 h. EGFR levels were quantified to that of α-tubulin. (E) LAMP-2A was required for oz-apraA–induced Hsp90 client protein degradation. A549 cells (top panels) or MDA-MB-453 cells (bottom panels) were transiently transfected with pSuper-LAMP-2A for 48 h and treated with 100 nM oz-apraA or 500 nM GA for 24 h. IP, immunoprecipitation; L2A, LAMP-2A.