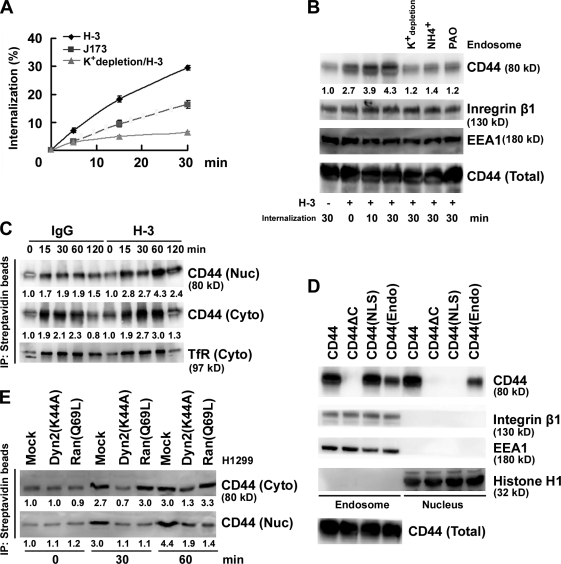
Figure 2.
Nuclear translocation of CD44 by endosomal sorting. (A) H1299 cells were incubated with biotin-conjugated anti-CD44 mAb H-3 and J173 at 4°C separately followed by further incubation at 37°C for 5, 15, and 30 min as indicated. Internalization was measured by flow cytometry. The percentage of internalization was calculated by setting the mean fluorescence intensity of cells after biotin labeling but without glutathione incubation as 100%. The value of each time point is shown as means ± SD from three independent experiments. (B) H1299 cells were cultured in complete medium with and without 50 mM NH4Cl or 20 µM phenylarsine oxide (PAO) or in K+ depletion medium at 37°C for 1 h followed by further incubation in the presence of H-3 for various times as indicated. Total cell lysates and endosomes purified by sucrose gradient centrifugation were subjected to Western blotting. (C) H1299 cells were surface labeled with biotin. Cytosolic (Cyto) and nuclear (Nuc) fractions were affinity purified with avidin-conjugated beads and analyzed by Western blotting. (D) Western blot analyses of endosomal and nuclear fractions in individual AZ521/CD44 cell clones. (E) Western blot analyses of cytosolic and nuclear fractions derived from H1299 cells transfected with and without plasmids encoding Dyn 2(K44A) or Ran(Q69L). (B, C, and E) The relative intensities of the bands, which were quantified by densitometry, are shown.