Abstract
Background
Bisphosphonates differ in their in vitro potency, avidity for bone, and rapidity of onset in clinical trials. To address potential differences between bisphosphonates in comparative effectiveness, we compared new users of alendronate and risedronate to determine if there were differences in the risk of clinical fractures at 1 year and beyond.
Methods
Using claims data from a U.S. health care organization, we identified new, adherent users of weekly alendronate or risedronate and assessed subsequent fractures. We calculated fracture incidence rate differences and ratios between the two agents.
Results
There were no significant differences in fracture rates between alendronate users (n=12,956) and risedronate users (n=6,107) at 1 year. Using all available data, the rate of hip fracture was higher among risedronate users compared to alendronate users (absolute rate difference approximately 5 per 1000 person-years). Risedronate users had a higher relative rate [RR] of hip fracture (RR = 1.77, 95% CI 1.15 – 2.74) and similar rates of clinical vertebral and nonvertebral fractures compared to alendronate users.
Conclusions
The absolute rate of clinical fractures among alendronate and risedronate users was similar both at one year and beyond, suggesting comparable effectiveness between agents.
Keywords: bisphosphonate, alendronate, risedronate, fracture, osteoporosis
Introduction
In the clinical trials that led to approval by the U.S. Food and Drug Administration, both alendronate and risedronate reduced the risk for clinical vertebral and non-vertebral fractures including hip fractures (1–5). A head–to-head study of weekly alendronate and risedronate users showed that alendronate users had greater increases in bone mineral density (BMD) and more suppression of bone turnover markers, but there were no significant differences in the risk of fracture (6). Indeed, the sample size and length of follow-up requirements of conducting a head-to-head bisphosphonate trial adequately powered to detect differences in the rate of non-vertebral fractures would lead to a very sizable logistic and financial burden for such a study. Despite these challenges, there is great interest in determining if there are potential differences between bisphosphonates in their ability to reduce fracture risk.
Two biologic factors that may be relevant in considering differences between bisphosphonates include their ability to bind tightly to hydroxyapetite in bone, and their ability to inhibit the farnesyl pyrophosphonate synthase (FPPS) enzyme (7–9), the putative target for bisphosphonate activity. Compared to the first approved oral bisphosphonate alendronate, risedronate has been shown to bind with less avidity but demonstrates greater inhibition of FPPS. Thus, it has been hypothesized that risedronate may be able to act more quickly to reduce fractures compared to alendronate. A recent observational study of 33,830 persons initiating weekly alendronate or risedronate demonstrated that compared to alendronate, the risedronate users achieved a significantly greater risk reduction of hip and non-vertebral fractures at 1 year compared to alendronate users (10).
We sought to use similar methods in a different population-base to determine if there were differences in the rate of clinical fractures between weekly risedronate and weekly alendronate users. Our analysis extended to three years and beyond to determine potential differences in fracture rates between agents later in time.
Methods
Data Source and Eligible Population
We used the administrative claims databases of a large commercial health care organization covering more than 20 million lives in 7 U.S. census regions. We identified women with medical and pharmacy benefits filling new prescriptions for weekly alendronate or risedronate from October 2000 to June 2005. Individuals with malignancy, HIV disease, and Paget’s disease of bone were excluded. New bisphosphonate (BP) users were defined as those persons initiating therapy after at least a six month period without any BP prescription. The date of the first filled BP prescription after this 6 month period was defined as the “index date”. Baseline demographic characteristics, comorbidities, and health services utilization were examined in the six months prior to the index date, stratified by which drug they initiated. We restricted the population to women at least 65 years old, since this was the target age group evaluated in the large bisphosphonate trials. We excluded women initiating the 35 mg/week alendronate dose used for prevention (9.9% of the original cohort) since we expected that this might denote women with less severe osteoporosis who would not be comparable to standard dose risedronate users.
Adherence with Bisphosphonates and Drug Exposure Time
Observation time began at the index date. Adherence with BPs was evaluated by examining gaps between prescriptions, and non-adherence was defined by more than a 15 day gap after the end of one prescription before the next prescription was filled. Observation time was censored at the first time of non-adherence, the fill date of a non-BP osteoporosis medication known to impact bone turnover (i.e. teriparatide, raloxifene, and calcitonin), disenrollment from the health plan, or the end of the study period. Switching BPs also censored observation time.
Outcome Assessment
The first occurrence of a fracture was the primary endpoint of the study. Similar to groupings used in BP clinical trials, fracture types were classified as hip; wrist/forearm; clinical vertebral; any non-vertebral (including hip, wrist/forearm, humerus, clavicle, pelvis, and leg); and non-hip, non-vertebral. Fractures were identified using International Classification of Diseases (ICD-9) codes and were required to appear on an evaluation and management (E/M) claim from a physician (as opposed to a claim from a diagnostic test or other procedure, which may represent a ‘rule out’ diagnosis). Individuals who had a fracture in the 180 days prior to first BP use were excluded from being at-risk for a fracture of that same type after the index date to avoid misclassifying follow-up visits for a recent fracture as an incident one. However, these individuals were not excluded from being at-risk for fractures of different types; for example, a hip fracture prior to the index date would exclude that individual from being at risk for another hip fracture but not a wrist fracture.
Statistical Analysis
We examined and compared the absolute incidence rates and incidence rate differences of hip, clinical vertebral, and non-vertebral fractures between new alendronate and risedronate users. We used Cox proportional hazards models to evaluate the crude and adjusted relationship between use of risedronate versus alendronate (the independent variable) and time to fracture (the dependent variable). Hazard ratios were computed as estimates of incidence rate ratios. Factors used to screen variables to be included in the Cox regressions were all those that were significant at p < 0.20 in bivariate analyses. Those that remained independently significant at p < 0.05, and variables of high clinical interest were included in the final models. Kaplan Meier curves graphically illustrated the differences in cumulative fracture incidence between users of each of the two medications and were compared using Wilcoxon tests.
Absolute fracture rates and rate differences were used to estimate a 1 year cumulative fracture incidence and compute cumulative incidence differences with the associated number needed to treat (NNT). The NNT represents the number of persons that would need to be treated with alendronate (rather than risedronate) for 1 year to prevent one fracture of that type. As part of a sensitivity analysis, we truncated observation time at one year to determine if fracture rates differed between agents in the first year after initiating therapy. All analyses were performed using SAS 9.1 (SAS Institute, North Carolina).
Results
The demographic characteristics, comorbidities, and health services utilization of the 19,063 women initiating weekly alendronate or risedronate are shown in Table 1. There were some differences in the demographics and other characteristics between users of the two agents with respect to age, comorbidities, prior medications and health service utilization. Although some of these imbalances were statistically significant, the numeric differences were small.
Table 1.
Demographic Characteristics, Comorbidities, and Health Services Utilization* of Women Initiating Weekly Alendronate or Risedronate and Adherent**
| Alendronate (n = 12,956) N (%) | Risedronate (n = 6,107) N (%) | P value | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age | < 0.0001 | ||
| 65–74 | 7488 (57.8) | 3287 (53.8) | |
| ≥ 75 | 5468 (42.2) | 2820 (46.2) | |
|
| |||
| Prior Fracture | |||
| Hip | 259 (2.0) | 133 (2.2) | 0.42 |
| Wrist/Forearm | 201 (1.6) | 73 (1.2) | 0.05 |
| Clinical Vertebral | 333 (2.6) | 172 (2.8) | 0.29 |
| Non-hip, non-vertebral | 486 (3.8) | 221 (3.6) | 0.65 |
| Any non-vertebral | 619 (4.8) | 294 (4.8) | 0.91 |
|
| |||
| Osteoporosis diagnosis | 5635 (43.5) | 2623 (43.0) | 0.48 |
| Other Selected Comorbidities | |||
| Diabetes | 1305 (10.1) | 701 (11.5) | 0.003 |
| Rheumatoid Arthritis | 386 (3.0) | 179 (2.9) | 0.55 |
| Hyperlipidemia | 4040 (31.2) | 1948 (31.9) | 0.32 |
| Tobacco abuse | 71 (0.6) | 49 (0.8) | 0.04 |
| Hyperthyroidism | 155 (1.2) | 72 (1.2) | 0.92 |
| Charlson Comorbidity Index, mean ± SD*** | 0.64 ± 1.2 | 0.70 ± 1.3 | 0.002 |
|
| |||
| Prior Use of Selected Medications | |||
| Systemic Estrogen | 1622 (12.5) | 752 (12.3) | 0.69 |
| Teriparatide | 4 (0.0) | 5 (0.1) | 0.13 |
| Raloxifene | 328 (2.5) | 160 (2.6) | 0.72 |
| Nasal calcitonin | 363 (2.8) | 166 (2.7) | 0.74 |
| Systemic Glucocorticoids | 1110 (8.6) | 589 (9.6) | 0.01 |
|
| |||
| Health Services Utilization* | |||
| Outpatient visits, mean ± SD | 3.5 ± 3.1 | 3.7 ± 3.3 | 0.0003 |
| Any hospitalization | 1049 (8.1) | 533 (8.7) | 0.14 |
| Bone Mineral Density Test | 5904 (45.6) | 3108 (50.9) | < 0.0001 |
| Other Screening Tests | |||
| Mammography | 3460 (26.7) | 1748 (28.6) | 0.01 |
| Colonoscopy | 610 (4.7) | 300 (4.9) | 0.54 |
| Fecal Occult Blood Test | 1794 (13.9) | 776 (12.7) | 0.03 |
| Flexible Sigmoidscopy | 63 (0.5) | 24 (0.4) | 0.37 |
The chi-square test was used to compare categorical variables and t-tests for continuous variables
all factors assessed in the 6 months prior to first bisphosphonate use
defined as having no more than a 15 day gap after the end of one prescription until the next prescription was filled
the Charlson comorbidity index is an aggregate sum of pre-defined medical comorbidities, from (16)
Figure 1 shows the fracture free survival curves for hip fracture, stratified by medication use. Despite a difference that was statistically significant (p = 0.03) and indicated a lower fracture rate among alendronate users, the survival curves were nearly superimposed. Substantial attrition in both cohorts occurred, predominantly due to the censoring of patients for non-adherence. Only approximately 22% and 3% of the original cohort remained at 1 and 3 years after the start of follow-up, respectively.
Figure 1.
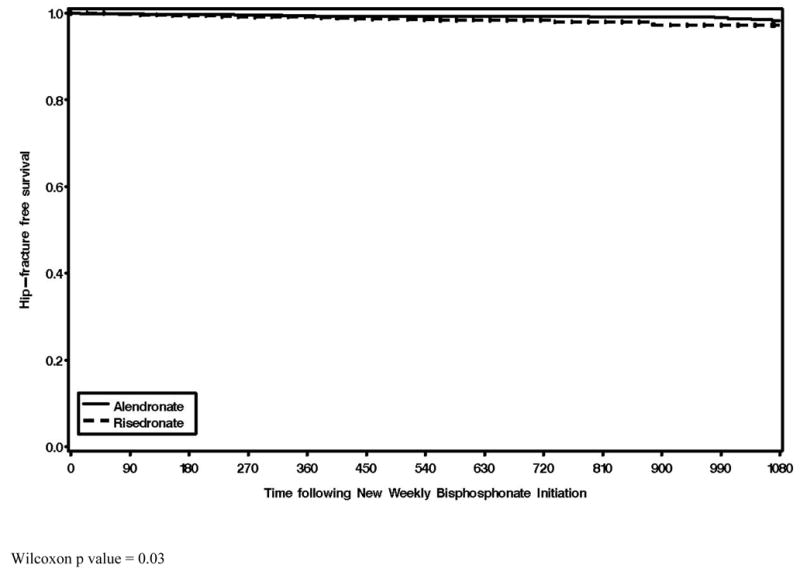
Hip Fracture-Free Survival Curves Comparing Persons Treated with Risedronate (dotted) or Alendronate (Solid)
Wilcoxon p value = 0.03
Table 2 shows the absolute incidence of fractures among users of alendronate and risedronate. The absolute rate differences between users of the two agents were small (approximately 5 per 1000 person years for both hip and nonvertebral fractures). Applying these rates to obtain cumulative fracture incidence after 1 year of treatment, the number that would need to be treated (NNT) for 1 year with alendronate compared to risedronate to prevent one hip or nonvertebral fracture was approximately 200 people.
Table 2.
Fracture Rates for Users of Alendronate or Risedronate
| N (absolute fracture incidence per 1,000 person years) | Crude and Adjusted Hazard Ratios | |||||||
|---|---|---|---|---|---|---|---|---|
| Alendronate | Risedronate | Incidence Difference | Number Needed to Treat* | Alendronate | Risedronate | |||
| Crude | Adjusted** | P value of adjusted result | ||||||
|
| ||||||||
| Fracture Type | ||||||||
| Hip | 47 (5.5) | 38 (11.1) | 5.5 | 180 | 1.0 | 1.99 (1.29 – 3.06) | 1.77 (1.15 – 2.74) | 0.01 |
| Clinical Vertebral | 54 (6.4) | 27 (7.9) | 1.5 | 667 | 1.0 | 1.18 (0.74 – 1.87) | 1.12 (0.70 – 1.79) | 0.64 |
| Non-vertebral | 165 (19.6) | 83 (24.3) | 4.7 | 214 | 1.0 | 1.25 (0.96 – 1.63) | 1.19 (0.91 – 1.56) | 0.20 |
Number needed to treat indicates the number of persons that would need to be treated with alendronate, rather than risedronate, for 1 year to preferentially prevent one fracture of that type
adjusted for age (as a continuous variable), number of outpatient visits, Charlson comorbidity index, use of screening tests including BMD, prior fracture, and use of prior estrogen, glucocorticoids, and non-bisphosphonate osteoporosis medications (used before initiating bisphosphonate therapy)
Our results were similar in a sensitivity analysis that truncated observation time at one year. The crude relative rate of hip fracture among risedronate users was 1.74 (1.08 – 2.81), which was not statistically significant after multi-variable adjustment (HR = 1.55, 95% CI 0.96 – 2.51). There were no significant differences between these two agents at one year in the rates for clinical vertebral or all non-vertebral fractures.
Conclusions
In this observational study of 19,063 women initiating weekly or risedronate therapy, we observed that absolute rates of hip, clinical vertebral, and non-vertebral fractures were very similar between the two agents, with a lesser rate of hip fracture among persons taking alendronate. Although the adjusted rate of hip fractures was 77% greater among the risedronate users compared to the alendronate users, the survival curve in Figure 1 illustrates that the alendronate and risedronate curves were almost superimposed, which illustrates the fact that the absolute difference in fracture rate between users of the two agents was small (approximately 5 fractures per 1,000 person-years). Additionally, our primary results were consistent with results from our sensitivity analysis that examined only the first year on therapy. Collectively, our data do not support an earlier non-vertebral fracture risk reduction by risedronate.
Despite a similar approach to the rigorous methods used in the study by Silverman et. al.(10), we were unable to replicate that study’s findings that showed an earlier onset of action of risedronate compared to alendronate in one year fracture risk. They demonstrated a cumulative incidence of hip fracture of approximately 0.5% at 1 year, which is similar to our hip fracture rate of 5.5 per 1000 person years in Table 2. However, they found an early and statistically significant benefit for risedronate compared to alendronate that we did not identify. Although the reasons for the discordance in their results and ours are unknown, our findings are consistent with data showing greater suppression of bone turnover and greater increases in BMD at two years in a randomized, placebo controlled trial of 1053 women (mean age 65 years) (6). This may account for our finding that the relative rate of hip fractures was lower among the alendronate users compared to the risedronate users. Moreover, with respect to the timing of the onset of action, another study showed a rapid and substantial suppression of bone turnover markers among alendronate patients as early as twelve weeks after initiating therapy (11). These reductions in bone resorption markers at twelve weeks were similar to the nadir reached at one year with continued use of alendronate(12).
The strengths of our study include evaluation of a large number of community-based persons initiating weekly bisphosphonate therapy. We were able to examine incidence rates and rate ratios of fractures using data from 1 and 3 years of observation and beyond, which allowed us to examine potential differences in the timing of fracture risk reduction related to the possible differential onset of action of the respective drugs. One potential limitation of our study is that we lacked medical record validation to confirm fractures. Particularly for spine fractures, misclassification of incident clinical vertebral fractures may be significant (13). Any potential misclassification would not be expected to differ between alendronate and risedronate users and most likely would bias our results towards the null hypothesis. Additionally, pharmacy claims data does not reflect actual medication taking behavior, and misclassification of exposure could bias the estimated rate ratios towards the null. However, we have previously shown high agreement between pharmacy data and actual medication taking behavior for bisphosphonates (14). Finally, we intentionally selected only those patients that were highly adherent patients in order to simulate a clinical trial. Although adherence with bisphosphonates is suboptimal overall (15) and maximizing medication adherence is critical to achieving optimal fracture protection, our exclusion of non-adherent patients has no impact on the internal validity of our results.
In summary, the RisedronatE and ALendronate Investigation over Three Years (REALITY) showed similar absolute rates of fracture between new users of the both agents. Modest differences in the relative rate of hip fracture that favored alendronate users may be of small clinical importance. Moreover, we found no early significant fracture risk reduction associated with either agent compared to the other. We conclude that the reduction in fracture rates is largely similar between users of these two medications.
Acknowledgments
Funding
Some of the investigators (JRC & KGS) receive salary support from the National Institutes of Health (AR053351, AR052361) and the Arthritis Foundation (JRC).
Disclosures:
JC: research grants: Novartis, Amgen, Merck, Proctor & Gamble, Eli Lilly; consulting: Roche; speakers bureau: Merck, Procter and Gamble, Eli Lilly, Roche
AW: research grants: Novartis
HC: research grants: Amgen
KGS: research grants : Novartis, Amgen, Aventis, Merck, Procter & Gamble, Eli Lilly, Roche; consulting or speaking: Merck, Proctor and Gamble, Eli Lilly, Roche, Novartis, Amgen
ED: research grants: Amgen
References
- 1.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. J Am Med Assoc. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 3.McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 4.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 5.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 6.Bonnick S, Saag KG, Kiel DP, et al. Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab. 2006;91(7):2631–7. doi: 10.1210/jc.2005-2602. [DOI] [PubMed] [Google Scholar]
- 7.Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296(2):235–42. [PubMed] [Google Scholar]
- 8.Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38(5):617–27. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Green JR, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9(5):745–51. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- 10.Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34. doi: 10.1007/s00198-006-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saag K, Lindsay R, Kriegman A, Beamer E, Zhou W. A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone. 2007;40(5):1238–43. doi: 10.1016/j.bone.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 12.McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41(1):122–8. doi: 10.1016/j.bone.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Curtis J, Mudano A, Solomon D, Xi J, Saag K. American Society for Bone and Mineral Research 2007. Honolulu, Hawaii: 2007. Validation of Vertebral Fractures using Administrative Claims Data. [Google Scholar]
- 14.Curtis JR, Westfall AO, Allison J, Freeman A, Kovac SH, Saag KG. Agreement and validity of pharmacy data versus self-report for use of osteoporosis medications among chronic glucocorticoid users. Pharmacoepidemiol Drug Saf. 2006;15(10):710–8. doi: 10.1002/pds.1226. [DOI] [PubMed] [Google Scholar]
- 15.Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21(9):1453–60. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]


