Abstract
Rationale
During the course of an infection, the pro-inflammatory cytokine tumor necrosis factor alpha (TNFα) acts in the brain to trigger development of behavioral responses, collectively termed sickness behavior. Biological activities of TNFα can be mediated by TNF receptor type 1 (TNF-R1) and type 2 (TNF-R2). TNFα activates neutral sphingomyelinase through the TNF-R1 adapter protein FAN (factor associated with neutral sphingomyelinase activation), but a behavioral role of FAN in the brain has never been reported.
Objectives
We hypothesized that TNFα-induced sickness behavior requires TNF-R1 and that FAN is a necessary component for this response.
Methods
We determined the role of brain TNF-R1 in sickness behavior by administering an optimal amount of TNFα intracerebroventricularly (i.c.v., 50 ng/mouse) to wild-type (WT), TNF-R1-, TNF-R2-and FAN-deficient mice. Sickness was assessed by decreased social exploration of a novel juvenile, induction of immobility and loss of body weight.
Results
TNF-R1-deficient mice were resistant to the sickness-inducing properties of i.c.v. TNFα whereas both TNF-R2-deficient and WT mice were fully responsive. Furthermore, the complete absence of TNFα-induced sickness behavior in FAN-deficient mice provided in vivo evidence that FAN-dependent TNF-R1 signaling is critical for this central action of TNFα.
Conclusions
This is the first report to demonstrate that TNFα-induced sickness behavior is fully mediated by TNF-R1 and that the adaptor protein FAN is a necessary intracellular intermediate for sickness behavior.
Keywords: Sickness behavior, TNF receptor, FAN, Ceramide, Gene deficiency, Mouse, Social exploration, Cytokine
Introduction
Activation of the innate immune system during the course of an infection induces a variety of physiological and behavioral changes known as sickness behavior, which includes decreased appetite, weight loss and reduced interest in the physical and social environment (Dantzer 2004b; Kelley et al. 2003; Kent et al. 1992b). Cytokine-induced sickness behavior is the expression of a motivational state that is triggered by immune stimuli and reorganizes the organism’s priorities (Dantzer 2004a; 2007). Since major depressive disorders can develop on a background of cytokine-induced sickness behavior, there has been a recent surge of interest in exploring interactions between activation of the innate immune system and psychopathology (Dantzer et al. 2008).
Tumor necrosis factor alpha (TNFα) plays a major role in inflammation and immune responses to infection. Its biologic activities are mediated by two structurally related but functionally distinct receptors belonging to the TNF-receptor (TNF-R) gene family, TNF-R1 and TNF-R2 (Vandenabeele et al. 1995). The intracellular domain of TNF-R1 contains several discrete regions that regulate cell survival, including death (DD), internalization (ID) and neutral sphingomyelinase (N-SMase, NSD) domains. Sphingomyelin (ceramide phosphocholine) is a major structural constituent of mammalian cell membranes that is generated upon SMase activation; it is crucial in the signal transduction pathways of TNFα [reviewed in (Kolesnick and Golde 1994; Kronke 1999)]. Activation of TNF-Rs increases intracellular ceramide by three pathways: neutral (N) and acidic (A) SMases that catalyze the breakdown of membrane sphingomyelin to ceramide and a de novo ceramide synthesis pathway. For instance, TNFα activates A-SMase through proteins that bind the TNF-R1 DD and N-SMase through the adapter protein FAN (factor associated with N-SMase). FAN binds specifically to the NSD of TNF-R1 (Adam-Klages et al. 1996; Adam et al. 1996; Kolesnick and Kronke 1998); FAN does not bind to TNR-R2. Fibroblasts from FAN-deficient mice are to resistance to TNFα-induced apoptosis and human fibroblasts transfected with mutated FAN are unable to activate N-SMase to generate ceramide in response to TNFα (Segui et al. 2001). N-SMase is expressed in the brain, where it can be activated by TNFα (Chakraborty et al. 1997; Zeng et al. 2005). These data provide strong evidence that FAN is required for many actions of TNFα that are mediated by TNF-R1, but its role and that of the downstream FAN protein in TNFα- induced behavioral changes has not been characterized.
High doses of lipopolysaccharide (LPS) cause endotoxic shock and death in wild-type (WT) mice, whereas mice devoid of TNF-R1 (Pfeffer et al. 1993; Rothe et al. 1993) are resistant and those mice lacking FAN are partially resistant to both LPS- and TNFα-induced endotoxemia (Malagarie-Cazenave et al. 2004). FAN deficiency increases survival following treatment with LPS, administered i.p., or TNFα, administered i.v., but the LPS-induced elevation in serum concentration of TNFα is not altered by FAN deficiency. LPS lethality is often a peripheral action that is associated with liver damage, but FAN-deficiency increased survival despite similar LPS-induced liver damage and serum levels of TNFα in control and FAN-deficient mice. The main difference between the control and FAN-deficient mice was a depressed hepatic ceramide/sphingomyelin ratio in FAN-deficient mice. It therefore appears that induction of ceramide is critical for some of the detrimental actions associated with an activated immune system. Despite the requirement of TNF-R1 and its downstream adaptor FAN for activation of N-SMase, it is unknown whether either are required for the induction of sickness behavior. Here we used mice devoid of either TNF-R1 or TNF-R2 to test the hypothesis that TNF-R1 is required for sickness responses. Optimal amounts of TNFα were directly injected into the cerebral lateral ventricles to target brain TNF-Rs. We next asked whether similar results could be obtained in another unique genetic model, mice that lack FAN. These experiments allow us to present direct evidence that TNF-R1, but not TNF-R2, mediates the sickness-inducing properties of TNFα in the brain and that this action requires the adapter protein FAN.
Material and methods
Genetic strains of mice
Mice carrying the TNFRsf1atm1Mak (TNF-R1−/−) (Pfeffer et al. 1993) and Tnfrsf1btm1Mum (TNF-R2−/−) (Erickson et al. 1994) mutations, respectively, were purchased from the Jackson Laboratory at 7 to 10 weeks of age (Bar Harbor, Maine, USA). Both strains have been backcrossed to C57BL/6J mice for at least 6 generations by null X null sib breeding. Heterozygotes were produced by crosses with wild type (WT) C57BL/6J mice and these animals were then interbred to produce TNF-R1−/− or TNF-R2−/− mice. Standard inbred WT C57BL/6J mice of 7 to 10 weeks of age (Bar Harbor, Maine, USA) were used as controls. FAN-deficient (FAN−/−) mice and their control counterparts, designated FAN+/+, of 7 to 10 weeks of age, backcrossed into the C57BL/6 strain, were obtained from heterozygous FAN+/− embryos. These mice were kindly provided by Drs. S. Adam and M. Krönke (Malagarie-Cazenave et al. 2004). Juvenile male mice (3–4 weeks of age) of the Crl:CD1(ICR)BR strain were used to provide stimuli for the social investigation behavioral paradigm. These juvenile mice were group-housed in cages (40 ×25 × 15 cm) in a different room. All animal protocols were approved by the Institutional Animal Care and Use Committees.
Animal housing
All mice were maintained under standard colony conditions in polycarbonate transparent cages on corn cob litter in a temperature- (23 ± 1 °C) and humidity- (40 %) controlled room maintained on a 12-12 h light/dark cycle (lights off at 09:00 h). Animals were provided free access to food and water. Test mice were group-housed for two weeks following surgery. Mice were then individually housed for 24 h prior to the first behavioral test and throughout the treatment period.
Surgical procedures
A stainless-steel guide cannula (23-gauge, 7 mm length) that was used for intracerebroventricular (i.c.v.) injections was surgically implanted unilaterally 1 mm above the lateral ventricle, as previously described (Johnson et al. 1997). A mixture of ketamine and xylazine (12.2 mg/kg and 1.8 mg/kg at 0.1 ml/10 g body weight, respectively) was injected intraperitoneally (i.p.) for anesthetization. Mice were then placed in a Kopf stereotaxic instrument (Tujunga, CA, USA) that was adjusted to the following coordinates for the guide cannula placement: 0.6 mm posterior and 1.5 mm lateral of the bregma to extend 2 mm below the skull surface at the point of entry (Paxinos and Franklin 2001). Mice were allowed to recover for 2 weeks prior to initiation of all behavioral tests. In order to confirm cannula placement in the lateral ventricles, a solution of India ink was injected into the guide cannula of at the end of each experiment. The brain of every mouse was then removed, sliced and the site of injection verified.
Treatments
Recombinant murine TNFα (R & D Systems, Minneapolis, MN) was dissolved in artificial cerebrospinal fluid (aCSF; NaHCO3 26.2 mM ; glucose 10 mM ; NaCl 120 mM ; Na2HPO4 1 mM ; KCl 2.5 mM ; MgCl2 1 mM ; CaCl2 2.5 mM). Based upon previous experiments (Bluthe et al. 2006), the optimal amount of TNFα required to induce sickness behavior in C57BL/6J mice was determined using four doses of TNFα: 0, 25, 50 and 100 ng/µl/mouse, where the dose of 0 corresponded to aCSF. LPS from Escherichia coli (serotype 127:B8, Sigma Chemical, St. Louis, MO) was dissolved in aCSF. The dose of 1 ng/µl/mouse of LPS to induce sickness behavior was based on previous experiments (Bluthe et al. 2000). All substances were administered i.c.v. to non-anesthetized animals by gravity through a 30-gauge needle over 90 s in a volume of one microliter. Each mouse received only one combination of treatments and aCSF (1 µl) was used as the control for i.c.v. injections.
Measurements of sickness behavior
Prior to initiation of all experiments, test mice were handled for 5 min everyday for 1 week. Beginning at 24 h prior to initiation of all experiments, test mice were individually housed in polycarbonate transparent plastic cages (26 ×20 × 14 cm). Behavioral tests were initiated during the dark phase. Behavioral observations were recorded using a video camera under red light illumination between 09:00 h and 17:00 h (dark phase). Prior to treatment, mice from each of the three strains were randomized according to both mean baseline social exploration time and body weight. Sickness behavior was measured as body weight loss (body weight pre-treatment – body weight 6 h after treatment), decreased duration of social exploration of a conspecific juvenile introduced for 4 min into the home cage of the mouse under test and the occurrence of immobility that is normally not displayed by mice during the social exploration test (Bluthe et al. 2006). Social exploration was carried out before and then 2, 6 and 24 h post i.c.v. injection using different juveniles on each occasion to provide a stronger stimulus for social investigation. All behavioral data were obtained by trained observers blinded to the genotype and treatment of experimental mice.
Experimental design and statistical analyses
All experiments were completely randomized designs and a factorial arrangement of treatments was used for experiments (3 strains × 2 treatments, n=6 per group). Data were analyzed by ANOVA. Within an experiment, mice were repeatedly tested for sickness behavior, so the statistical analysis involved mixed (repeated) measures. Duration of exploration and immobility were analyzed by a three-way ANOVA with strain (WT, TNF-R1−/− and TNF-R2−/− or FAN−/− and FAN+/+) and treatment (aCSF and TNFα or aCSF, LPS and TNFα) as the between-subject factors and time (0, 2, 6 and 24 h) as a within-subject factor. Body weight change was analyzed by a two-way ANOVA with strain (WT, TNFR1−/− and TNF-R2−/− or FAN−/− and FAN+/+) and treatment (aCSF and TNFα or aCSF, LPS and TNFα) as the between-subject factors. Post-hoc comparisons of individual group means were carried out by the protected least significant difference test. All results were summarized and presented as the mean ± SEM.
Results
TNF-R1 Mediates sickness behavior induced by central TNFα
We first determined the optimal amount of central TNFα that induces sickness behavior in C57BL/6J mice because both TNF-R1−/− and TNF-R2−/− mice are maintained on this genetic background. A dose-response experiment was conducted using i.c.v. administration of TNFα that previously had been found to be effective in others strains of mice (Bluthe et al. 2006; Bluthe et al. 2000). We found that 50 ng/µl TNFα administered i.c.v. is the optimal amount that induces a full spectrum of sickness behavior in mice (see Supplemental Material).
By using mice deficient for the type I interleukin-1 receptor (IL-1RI), we previously reported that abrogation of IL-1β activity via this receptor blocks the sickness-inducing action of i.c.v. IL-1β but not that of the cytokine inducer LPS (Bluthe et al. 2000). The sickness-inducing properties of LPS were mediated by endogenous production of TNFα in the brain since sickness was abrogated by pretreatment with a TNFα antagonist in the brain (Bluthe et al. 2000). However, even though TNFα is well-established to induce sickness behavior in inbred (Bluthe et al. 1991; Bluthe et al. 1994) and outbred (Bluthe et al. 2000) strains of mice, the specific receptor in the brain that mediates TNFα-induced sickness behavior remains unknown. To address this issue, three groups of mice were tested: WT, TNFR1−/− and TNF-R2−/−. Body weight (Table 1A) was significantly reduced 6 h post-treatment (treatment×strain interaction, F(2, 30) = 3.3, p<0.05). Post-hoc comparisons of treatment means revealed a reduction in body weight for only the WT (p<0.05) and TNF-R2−/− (p<0.01) mice that were injected with TNFα i.c.v. TNF-R1−/− mice did not differ from aCSF-treated mice.
Table 1.
Body weight changes in TNF-R-deficient and FAN-deficient mice induced by i.c.v. TNFα or LPS.
| A. TNF-R |
Δ Body Wt. (g) |
|
|---|---|---|
| WT | aCSF | 0.62 ± 0.18 |
| TNFα (50 ng) | −0.47 ± 0.42 * | |
| TNF-R1−/− | aCSF | −0.20 ± 0.55 |
| TNFα (50 ng) | 0.41 ± 0.29 | |
| TNF-R2−/− | aCSF | 0.09 ± 0.31 |
| TNFα (50 ng) | −1.23 ± 0.25 § | |
| B. FAN |
||
| FAN+/+ | aCSF | 0.27 ± 0.08 |
| TNFα (50 ng) | −0.97 ± 0.40 * | |
| LPS (1 ng) | −2.01 ± 0.46 # | |
| FAN−/− | aCSF | 1.16 ± 0.29 |
| TNFα (50 ng) | 1.15 ± 0.20 | |
| LPS (1 ng) | −2.22 ± 0.17 # |
Body weight changes 6 h after i.c.v. treatment. Values represent means ± SEM (n=6/group;
p<0.05
p<0.01
p<0.001 compared to the appropriate aCSF control).
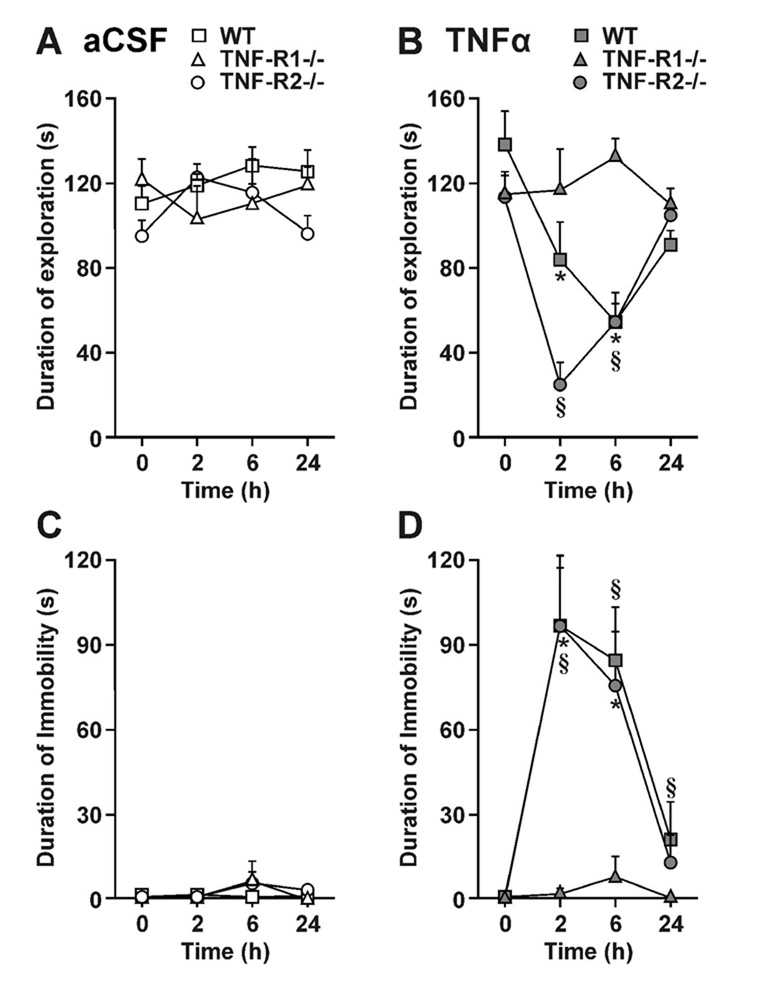
Duration of baseline social exploration ranged from 95 ± 8 s to 137 ± 15 s for the six treatments (time 0 in Fig. 1A, B). As expected, duration of social exploration remained stable following i.c.v. aCSF in the three strains, WT, TNF-R1−/− and TNF-R2−/− (Fig. 1A). In contrast, TNFα reduced duration of social exploration in a strain- [F(1, 15) = 6.3, p<0.01], time- [F(3, 45) = 14.0, p<0.001] and strain × time- [F(3, 45) = 8.2, p<0.001] dependent manner. Post-hoc comparisons of individual group means revealed that TNFα reduced duration of social exploration at 2 and 6 h for both WT (p<0.05) and TNFR2−/− mice (p<0.01). However, social exploration of TNF-R1−/− mice was not altered by TNFα.
Fig 1.
TNF-R1 mediates sickness behavior induced by central TNFα. WT, TNF-R1−/− and TNF-R2−/− mice were injected i.c.v. with either aCSF (A and C) or 50 ng of TNFα (B and D) in a volume of one microliter. Sickness behavior was evaluated at time 0 and 2, 6 and 24 h post-treatment during 4 min observation periods by measuring duration of social exploration (A and B) and immobility (C and D). Each value represents the mean ± SEM. (n=6/group; * p<0.05 or § p<0.01 compared to the aCSF control at each time).
There was no immobility at time zero in the three groups of mice (WT, TNF-R1−/− and TNFR2−/−). Little or no immobility was detected after i.c.v. aCSF (Fig. 1C). However, TNFα induced immobility in a strain- [F(2, 15) = 8.8, p<0.01], time- [F(2, 30) = 18.1, p<0.001], and strain ×time- [F(4, 30) = 4.1, p<0.01] dependent manner (Fig. 1D). Post-hoc comparisons of individual group means revealed that TNFα increased duration of immobility at 2, 6 and 24 h for both WT (p<0.01) and TNFR2−/− mice (p<0.01). However, TNF-R1−/− mice did not display any increase in immobility in response to central TNFα. These results clearly establish that TNF-R1, but not TNF-R2, mediates the ability of central TNFα to induce sickness behavior.
Sickness behavior induced by TNFα binding to TNF-R1 is specifically absent in FAN−/− mice
A crucial function of sphingomyelin, a major structural constituent of mammalian cell membranes, is to serve in the signal transduction cascade initiated by binding of TNFα to TNF-R1 (Kronke 1999). To determine whether FAN-mediated signal transduction is required for the sickness-inducing properties of TNFα, we tested the response of FAN-deficient mice to i.c.v. TNFα. A central injection of LPS was used as a positive control to ensure that both strains of mice were capable of expressing sickness behavior. The experiment was designed according to a 2 × 3 factorial arrangement of treatments (two strains, FAN+/+ and FAN−/−; three treatments: aCSF (1 µl), LPS (1 ng/µl, 1 µl), TNFα (50 ng/µl, 1 µl) (n=6 per group). Body weight (Table 1B) was significantly altered 6 h following i.c.v. treatment in a strain-dependent manner (treatment × strain interaction; F(2, 30) = 7.6, p<0.01). LPS induced a loss of body weight in both FAN+/+ and FAN−/− mice (p<0.01). However, i.c.v. TNFα (p<0.05) induced body weight loss in only the FAN+/+ mice.
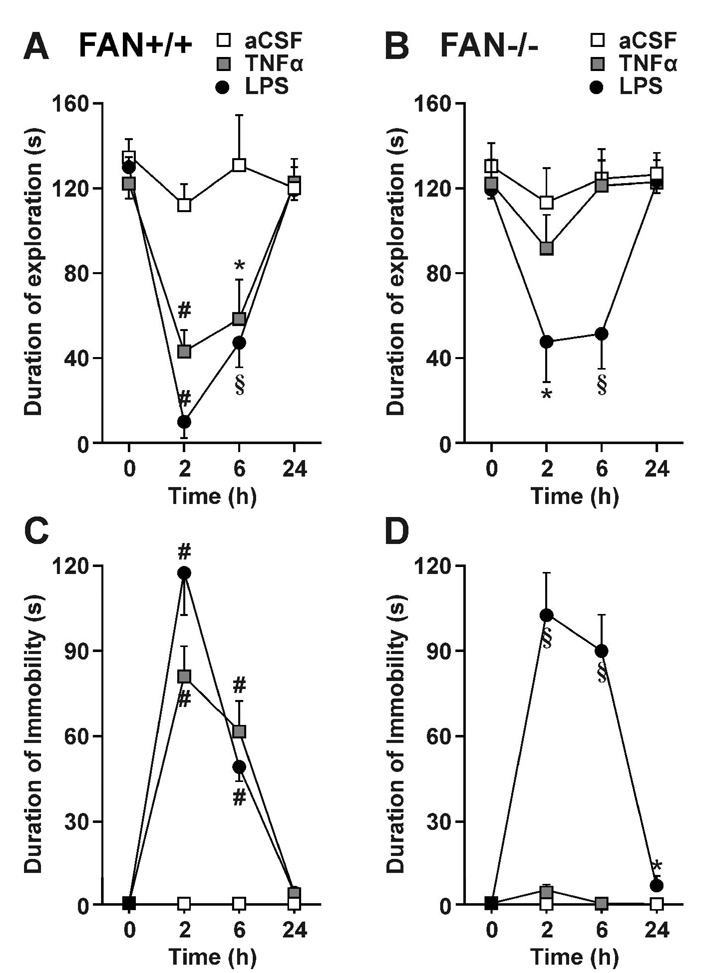
In the six treatment groups, baseline social exploration of a novel juvenile ranged from 120 ± 4 s to 135 ± 9 s (time 0 in Fig. 2A, B). To simplify description of results, duration of social exploration was analyzed independently for each strain of mouse, after the global analysis of variance had revealed a significant strain × treatment × time interaction. With the FAN+/+ mice, duration of social exploration (Fig. 2A) varied according to treatment [F(2, 15) = 14.2, p<0.001], time [F(3, 45) = 26.1, p<0.001] and the treatment × time interaction [F(6, 45) = 5.4, p<0.001]. Post-hoc comparisons of individual group means revealed that both i.c.v. LPS and TNFα treatments reduced duration of social exploration at 2 and 6 h (p<0.01) with FAN+/+ mice. Similar results were obtained in FAN−/− mice, with the important exception that these mice no longer responded to i.c.v. TNFα (Fig. 2B). Duration of social exploration varied according to treatment [F(2, 15) = 4.0, p<0.05], time [F(3, 45) = 12.7, p<0.001] and a treatment × time interaction [F(6, 45) = 4.6, p<0.001]. Only the LPS treatment was able to reduce duration of social exploration at 2 and 6 h (p<0.01) with the FAN−/− mice.
Fig 2.
Sickness behavior induced by central TNFα is absent in mice that lack FAN, an adapter that links TNR-R1 activation to N-SMase activation. FAN+/+ (A and C) and FAN−/− (B and D) mice were treated i.c.v. with one microliter of aCSF, LPS (1 ng) or TNFα (50 ng) and sickness behavior was evaluated during 4 min observation periods at time 0 and 2, 6 and 24 h post-treatment by measuring duration of social exploration (A and B) and immobility (C and D). Each value represents the mean ± SEM. (n=6/group; * p<0.05, § p<0.01 or # p<0.001 compared to aCSF at each time).
At baseline, both FAN+/+ and FAN−/− mice were fully active. After treatment, duration of immobility of FAN+/+ mice varied according to treatment [F(2, 15) = 37.2, p<0.001], time [F(2, 30) = 75.4, p<0.001] and the treatment × time interaction [F(4, 30) = 22.9, p<0.001]. Post-hoc comparisons revealed that both i.c.v. LPS and TNFα treatments increased immobility at 2 and 6 h (Fig. 2C). With FAN−/− mice, mean duration of immobility varied according to treatment [F(2, 15) = 56.5, p<0.001], time [F(2, 30) = 28.7, p<0.001] and the treatment × time interaction [F(4, 30) = 26.4, p<0.001] (Fig. 2D). LPS caused immobility at 2 and 6 h (p<0.01), and a modest residual immobility even at 24 h. In contrast, the FAN−/− mice remained active despite i.c.v. TNFα injection. These data unequivocally demonstrate that FAN-mediated signal transduction is essential for inducing sickness behavior in mice injected with TNFα into the lateral ventricles of the brain.
Discussion
Several groups of investigators have established that central injections of TNFα induce sickness behavior, but whether sickness depends on activation of one or both isoforms of TNF receptors remain unknown. Data from our experiments now unequivocally establish that mice lacking TNF-R1, but not TNF-R2, do not develop any of the TNFα-induced signs of sickness behavior, i.e., weight loss, reduction in social investigation and immobility. The ability of TNF-R2-deficient mice to fully respond to central TNFα is entirely consistent with results that we recently reported (Palin et al. 2007). Collectively, these new observations are compatible with three conclusions: (a) TNF-R1 mediates the sickness-inducing properties of TNFα administered into the brain, (b) all three signs of sickness behavior require activation of TNF-R1-mediated signaling, and (c) TNF-R2 does not protect against i.c.v. infusion of TNFα, as shown by nearly equivalent sickness responses of WT and TNF-R2−/− mice. Finally, of the many downstream events triggered by binding of TNFα to TNF-R1, we now implicate FAN activation in sickness behavior. In the present experiments, central administration of TNFα was unable to induce any symptoms of sickness in FAN−/− mice. This important finding suggests that TNF-R1-dependent, FAN-regulated activation of the sphingomyelin-ceramide pathway is required for brain TNFα to induce sickness behavior.
The role of TNFα in the brain has a storied history because it has been reported to have both beneficial and damaging biological properties in the central nervous systm (Correale and Villa 2004). At least some of these discrepancies may be due to the concentration of TNFα that was used, as it has been recently reported that high concentrations of TNFα acting via TNF-R1 potentiate, whereas lower concentrations acting via TNF-R2 protect, against neuronal excitotoxicity (Bernardino et al. 2005). There are also likely to be regional-specific effects of TNFα. For example, mice deficient in TNFα display a reduction in arborization of apical dendrites in the CA1 and CA3 regions (Golan et al. 2004). These TNFα-deficient mice have improved spatial memory, which is in agreement with our recent findings that an inhibitor of TNFα significantly impairs the ability of the neurotoxin kainate to cause loss of spatial memory (Bluthe et al. 2005). Similarly, using TNF-R knockout mice like those reported here, it was concluded that TNF-R1 inhibits proliferation of adult hippocampal progenitors (Iosif et al. 2006). These findings are in accord with growing evidence which indicates that inhibition of TNFα synthesis and activity in the brain would be beneficial for developing therapeutics to treat or protect against neurodegenerative diseases (Greig et al. 2004).
Given the strong evidence for a crucial role for TNFα in causing sickness behavior, our objective was to elucidate the molecular mechanisms that are required by TNFα to elicit sickness behavior. By administering TNFα i.c.v., we were able to overcome major problems of interpretation that might be caused by lack of expression of TNF-R outside the brain. Following i.c.v. injection of recombinant human (rHu)TNFα, we have already demonstrated the induction of sickness behavior in mice (Bluthe et al. 1991; Bluthe et al. 1994). Indeed, in the mouse system, rHuTNFα behaves as a relatively pure agonist of the murine TNF-R1 since it does not bind to TNF-R2 (Lewis et al. 1991). Therefore, we hypothesized that the central effect of TNFα might be exclusively mediated by TNF-R1. Using mouse models with genetic deletions of either TNF-R1 or TNF-R2, we demonstrate (Fig. 1, Table 1A) that central injection of TNFα induces weight loss, decreases duration of social exploration and increases immobility in both WT and TNF-R2−/− mice. Peak reductions in social exploration occurred slightly earlier (Fig. 1B) and body weight loss (Table 1A) was greater in TNF-R2−/− mice injected with central TNFα compared to WT mice, but time spent immobile was identical at all time points in these two strains (Fig. 1D). Therefore, we found little evidence that TNF-R2 protects against sickness behavior in this model and no evidence that it mediates sickness behavior. Conversely, TNF-R1−/− mice remained totally refractive to central injection of TNFα, regardless of the form of sickness behavior that was measured. This finding establishes that TNF-R1 in the brain is a common molecular messenger that is required for i.c.v.-injected TNFα to induce all three of these symptoms of sickness. This is the first demonstration that TNF-R1 mediates symptoms of TNFα-induced sickness behavior.
The intracellular mechanisms mediating the actions of TNFα/TNF-R1 on sickness behavior in vivo are not known. Since the last decade, several intracellular events by which TNF-R1 transmits signals through the cell membrane into the cytoplasm and nucleus following binding of TNFα have been elucidated (Vandenabeele et al. 1995). There are two major pathways involving phospholipases, A-SMase and N-SMase pathways, and each pathway leads to different cellular responses (Wiegmann et al. 1994). TNFα stimulation of N-SMase via FAN has been linked to its apoptotic properties in human and murine fibroblasts (Segui et al. 2001). Using an in vitro cell culture system, we have shown that the TNFα-sphingomyelinase-ceramide pathway, acting via N-SMase, mediates the inhibitory properties of TNFα on protein synthesis in murine muscle progenitors (Strle et al. 2004). Based upon these results, we hypothesized that loss of TNF-R1-associated FAN would prevent central TNFα from inducing sickness behavior. In this report, we demonstrate (Fig. 2, Table 1B) that central injection of TNFα induces weight loss, reduces social exploration and increases immobility in the FAN+/+ mice. However, consistent with our hypothesis, FAN−/− mice were refractive to the central injection of TNFα, regardless of the form of sickness behavior that was measured. To our knowledge, this is the first demonstration that the adaptor protein FAN mediates symptoms of sickness behavior caused by intracerebral injection of TNFα.
Sickness behavior that occurs during the course of infection has also been found to occur in rodents following systemic or central administration of either of the two major pro-inflammatory cytokines, interleukin (IL)-1β and TNFα (Bluthe et al. 1991; Bluthe et al. 2000; Bluthe et al. 1994). Cytokines injected into the lateral ventricles of the brain induce sickness behaviors at roughly 100-to 200-fold lower concentrations that those that are active in the periphery (Kent et al. 1992a). The fact that the symptoms of sickness almost disappear within 24 h after cytokine administration supports a causal role for cytokines in the mediation of this condition. Critical to the development of therapeutic approaches to increase quality of life is defining the role of pro-inflammatory cytokines, in particular TNFα, in the induction of such a sickness behavior [reviewed in (Kelley et al. 2003)]. Unfortunately, the molecular mechanisms by which TNFα induces sickness behavior remain unknown, although the site of action of TNFα is within the central nervous system (Kelley et al. 2003; Konsman et al. 2002). Experiments in this report clearly identify brain TNF-R1 and its downstream adaptor protein FAN as critical molecular elements that are required for central administration of TNFα to induce sickness behaviors.
The major symptoms of sickness behavior, including decreased motivation to eat as well as social and exploratory behavior, are reminiscent of those observed in depressed patients (Dantzer 2004b; Dantzer et al. 2006; Dantzer et al. 2008). There is evidence that in vulnerable individuals these core symptoms of sickness behavior can eventually culminate in the development of depressive-like behavior (Capuron and Dantzer 2003; Raison et al. 2006). This event is associated with a decrease in plasma tryptophan as a consequence of activation of indoleamine 2, 3-dioxygenase that degrades tryptophan along the kynurenine pathway (O’Connor et al, 2008). TNFα is likely to be involved since it increases the activity of this enzyme both in the periphery (Schiller et al. 1991) and in the brain (Daubener et al. 1996). Although N-SMase has not yet been shown to be involved in clinical depression, there is already evidence that A-SMase activity and ceramide concentrations are higher in peripheral blood mononuclear cells of patients with major depressive episodes (Kornhuber et al. 2005). Therefore, the present results are important because, independently of the traditional research of the neurotoxic or survival function of both TNF receptors, these data point to a crucial role of TNF-R1, SMases and ceramide in sickness behavior.
Sickness behavior is now a well-established physiological response to infection and it has been documented in all animal species that have been studied (Dantzer 2007), ranging from insects (Adamo 2006; Riddell and Mallon 2006) to humans (Raison et al. 2006). Substantial progress has been made in defining sickness behaviors and establishing a role for proinflammatory cytokines such as IL-1β and TNFα in inducing these behaviors. Indeed, sickness behavior can be used as a tool to develop pharmaceuticals to improve quality of life during illness as well as recovery from those illnesses (Kent et al. 1992b). In particular, there are similarities between some sickness symptoms and those observed in terminal cancer patients, such as fatigue, lack of motivation to eat and reduced interest in day-to-day activities (Kelley et al. 2003). All of these symptoms affect the cancer patient’s quality of life. Clearly, a better understanding of how cytokines, particularly TNFα, act in the brain is needed to improve the psychological well-being of cancer patients. Our present findings highlight this issue by demonstrating that major symptoms of sickness behavior induced by central TNFα are mediated by TNF-R1 and require signaling through its downstream adaptor protein FAN.
Supplementary Material
Acknowledgments
This research was supported by grants from National Institutes of Health to K.W.K. (MH 51569 and AG 029573) and R.D. (MH 071349). The authors gratefully acknowledge Caroline Nevoit for her excellent technical assistance.
References
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Kronke M. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. doi: 10.1016/s0092-8674(00)80169-5. [DOI] [PubMed] [Google Scholar]
- Adam D, Wiegmann K, Adam-Klages S, Ruff A, Kronke M. A novel cytoplasmic domain of the p55 tumor necrosis factor receptor initiates the neutral sphingomyelinase pathway. J Biol Chem. 1996;271:14617–14622. doi: 10.1074/jbc.271.24.14617. [DOI] [PubMed] [Google Scholar]
- Adamo SA. Comparative psychoneuroimmunology: evidence from the insects. Behav Cogn Neurosci Rev. 2006;5:128–140. doi: 10.1177/1534582306289580. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, Oliveira CR, Vezzani A, Malva JO, Zimmer J. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci. 2005;25:6734–6744. doi: 10.1523/JNEUROSCI.1510-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor alpha in mice. Eur J Pharmacol. 1991;209:281–283. doi: 10.1016/0014-2999(91)90184-r. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Frenois F, Kelley KW, Dantzer R. Pentoxifylline and insulin-like growth factor-I (IGF-I) abrogate kainic acid-induced cognitive impairment in mice. J Neuroimmunol. 2005;169:50–58. doi: 10.1016/j.jneuroim.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav Immun. 2006;20:57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–4456. [PubMed] [Google Scholar]
- Bluthe RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology. 1994;19:197–207. doi: 10.1016/0306-4530(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17 Suppl 1:S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Ziemba S, Drivas A, Ledeen RW. Myelin contains neutral sphingomyelinase activity that is stimulated by tumor necrosis factor-alpha. J Neurosci Res. 1997;50:466–476. doi: 10.1002/(SICI)1097-4547(19971101)50:3<466::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Correale J, Villa A. The neuroprotective role of inflammation in nervous system injuries. J Neurol. 2004;251:1304–1316. doi: 10.1007/s00415-004-0649-z. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004a;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Innate immunity at the forefront of psychoneuroimmunology. Brain Behav Immun. 2004b;18:1–6. doi: 10.1016/j.bbi.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Castanon N, Kelley KW, Konsman JP, Laye S, Lestage J, Parnet P. Cytokines, sickness behavior and depression. In: Ader R, editor. Psychoneuroimmunology. Fourth Edition. New York, NY: Academic Press; 2006. pp. 281–318. [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubener W, Remscheid C, Nockemann S, Pilz K, Seghrouchni S, Mackenzie C, Hadding U. Anti-parasitic effector mechanisms in human brain tumor cells: role of interferon-gamma and tumor necrosis factor-alpha. Eur J Immunol. 1996;26:487–492. doi: 10.1002/eji.1830260231. [DOI] [PubMed] [Google Scholar]
- Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KC, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- Golan H, Levav T, Mendelsohn A, Huleihel M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex. 2004;14:97–105. doi: 10.1093/cercor/bhg108. [DOI] [PubMed] [Google Scholar]
- Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, Rogers JT, Ovadia H, Lahiri DK. New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists. Ann N Y Acad Sci. 2004;1035:290–315. doi: 10.1196/annals.1332.018. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW, Gheusi G, Segreti S, Dantzer R, Kelley KW. C3H/HeJ mice are refractory to lipopolysaccharide in the brain. Brain Res. 1997;752:219–226. doi: 10.1016/s0006-8993(96)01454-0. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17 Suppl 1:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992a;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992b;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Medlin A, Bleich S, Jendrossek V, Henkel AW, Wiltfang J, Gulbins E. High activity of acid sphingomyelinase in major depression. J Neural Transm. 2005;112:1583–1590. doi: 10.1007/s00702-005-0374-5. [DOI] [PubMed] [Google Scholar]
- Kronke M. Involvement of sphingomyelinases in TNF signaling pathways. Chem Phys Lipids. 1999;102:157–166. doi: 10.1016/s0009-3084(99)00084-5. [DOI] [PubMed] [Google Scholar]
- Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci U S A. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagarie-Cazenave S, Segui B, Leveque S, Garcia V, Carpentier S, Altie MF, Brouchet A, Gouaze V, Andrieu-Abadie N, Barreira Y, Benoist H, Levade T. Role of FAN in tumor necrosis factor-alpha and lipopolysaccharide-induced interleukin-6 secretion and lethality in D-galactosamine-sensitized mice. J Biol Chem. 2004;279:18648–18655. doi: 10.1074/jbc.M314294200. [DOI] [PubMed] [Google Scholar]
- Palin K, Bluthe RM, McCusker RH, Moos F, Dantzer R, Kelley KW. TNFalpha-induced sickness behavior in mice with functional 55 kD TNF receptors is blocked by central IGF-I. J Neuroimmunol. 2007;187:55–60. doi: 10.1016/j.jneuroim.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. second edition (Deluxe) edn. New York: Academic Press; 2001. [Google Scholar]
- Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell CE, Mallon EB. Insect psychoneuroimmunology: immune response reduces learning in protein starved bumblebees (Bombus terrestris) Brain Behav Immun. 2006;20:135–138. doi: 10.1016/j.bbi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Storer BE, Witt PL, Alberti D, Tombes MB, Arzoomanian R, Proctor RA, McCarthy D, Brown RR, Voss SD, et al. Biological and clinical effects of intravenous tumor necrosis factor-alpha administered three times weekly. Cancer Res. 1991;51:1651–1658. [PubMed] [Google Scholar]
- Segui B, Cuvillier O, Adam-Klages S, Garcia V, Malagarie-Cazenave S, Leveque S, Caspar-Bauguil S, Coudert J, Salvayre R, Kronke M, Levade T. Involvement of FAN in TNF-induced apoptosis. J Clin Invest. 2001;108:143–151. doi: 10.1172/JCI11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 2004;145:4592–4602. doi: 10.1210/en.2003-1749. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Zeng C, Lee JT, Chen H, Chen S, Hsu CY, Xu J. Amyloid-beta peptide enhances tumor necrosis factor-alpha-induced iNOS through neutral sphingomyelinase/ceramide pathway in oligodendrocytes. J Neurochem. 2005;94:703–712. doi: 10.1111/j.1471-4159.2005.03217.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.