Abstract
We report one-pot synthesis, encapsulation, and solubilization of high-quality quantum dots based on the use of amphiphilic and multidentate polymer ligands. In this “all-in-one” procedure, the resulting QDs are first capped by the multidentate ligand, and are then spontaneously encapsulated and solubilized by a second layer of the same multidentate polymer upon exposure to water. In addition to providing better control of nanocrystal nucleation and growth kinetics (including resistance to Ostwald ripening), this procedure allows for in-situ growth of an inorganic passivating shell on the nanocrystal core, enabling one-pot synthesis of both type-I and type-II core-shell QDs with tunable light emission from visible to near-infrared wavelengths.
Semiconductor quantum dots (QDs) are nanometer-sized particles with unique optical and electronic properties, and are currently under intensive research for a broad range of applications such as solar energy conversion and molecular and cellular imaging.1–3 Significant advances have been made in the chemical synthesis of highly crystalline and monodispersed QDs, especially with the use of organometallic and chelated cadmium precursors,4,5 noncoordinating solvents,6 and inorganic passivating shells.7 However, the resulting nanocrystals are often hydrophobic and must be encapsulated and solubilized for many important applications. Aqueous synthetic procedures have been used as alternative approaches to prepare water soluble QDs, using small thiol-containing molecules or polymers with carboxylic acid functional groups as stabilizing agents.8–10 But these methods do not yield QDs with the fluorescence brightness or size monodispersity that are often achieved with the high-temperature organic procedures.
Here we report an “all-in-one” strategy for simultaneous synthesis, encapsulation, and solubilization of high-quality quantum dots. This one-pot method is based on the use of amphiphilic multidentate ligands and noncoordinaging solvents such as low-molecular weight polyethylene glycols (PEG) (MW = 350 Daltons). The multidentate polymer ligands contain aliphatic chains and carboxylic acid functional groups, and are found to act as both a cadmium precursor ligand and a nanoparticle surface stabilizer, leading to improved control of chemical reaction kinetics and increased resistance to Ostwald ripening. When exposed to water, excess polymer molecules spontaneously encapsulate and solubilize the QDs without any additional materials or steps. Furthermore, this synthetic procedure allows for in-situ growth of an inorganic passivating shell on the nanocrystal core, enabling one-pot synthesis of both type-I and type-II core-shell QDs.11
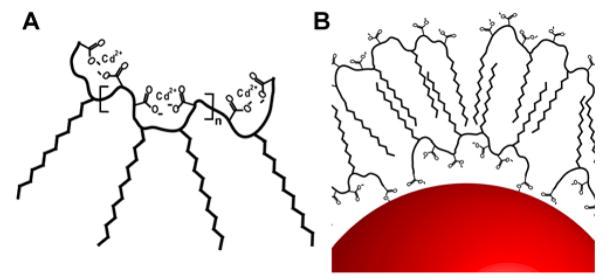
Figure 1 shows schematic structures of the multidentate polymer ligands for one-pot QD synthesis and the self-encapsulated QDs. A key intermediate is a cluster of chelated cadmium ions, generated by dissolving the amphiphilic polymer and cadmium oxide or cadmium acetate in noncoordinating polyethylene glycols at elevated temperatures. The reactivity of this clustered cadmium precursor plays an important role in controlling the nucleation and growth kinetics of nanocrystals. By increasing the length of the polymer backbone and the density of hydrophobic side chains, a dramatic steric hindrance effect comes into play resulting in homogeneous nucleation and growth, whereas the use of traditional monovalent ligands leads to uncontrollable and heterogeneous reactions (data not shown). By optimizing the balance between the hydrophobic and hydrophilic segments, the resulting QDs are spontaneously solubilized by a second layer of the same amphihphilic polymer when the reaction mixture is exposed to water (see Figure 1b). However, if the hydrophobic grafting percentage is too high, the number of surface carboxylic acid functional groups becomes too low for water solubilization. We have found that a 40 percent graft percentage (that is, 40% of the carboxylic acid groups are modified with hydrophobic 12-carbon aliphatic size chains) is nearly optimal for controlled nanoparticle growth and for solubilizing the capped QDs in water.
Figure 1.
(A) Schematic structure of the amphiphilic multidentate ligand with multiple chelated cadmium ions. (B) Diagram showing multidentate ligand binding to the QD surface. The resulting nanocrystals are spontaneously encapsulated and solubilized by a second layer of the same multidentate polymer upon exposure to water. See text for detailed discussion.
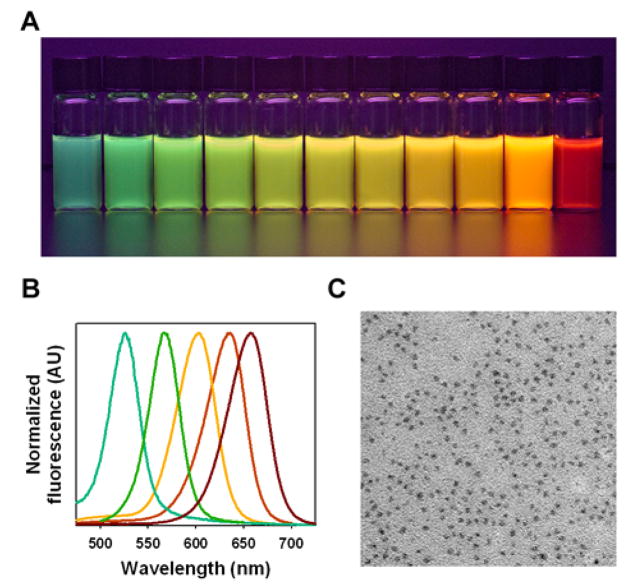
This improved control in reaction kinetics allows more precise tuning of the nanoparticle size and fluorescence emission wavelength across a wide range (Figure 2a). In fact, QD fluorescence emission can be consistently controlled within 1–2 nm. This high precision will become important as QDs are increasingly adopted for multiplexed biological and clinical assays, where consistency and repeatability are critical. The use of multidentate polymer precursors also provides a new route to ultrasmall QDs; for example, small CdTe cores emitting in the green range (515 – 525 nm) can be synthesized with narrow size distributions, allowing for a very large dynamic range from the green to the far red wavelengths (Figure 2a). It is worth noting that small QDs are often difficult to synthesize with traditional monovalent precursors because of the problems with kinetic control and Ostwald ripening. In contrast, the QDs capped with multidentate ligands are strongly resistant to Ostwald ripening. In fact, each polymer has approximately 15 carboxylic acid functional groups that are capable of coordinating with surface atoms. By increasing the overall binding affinity through multivalent interactions, the polymer capping can better stabilize small nanoparticles. However, some ripening does occur at increased temperatures after long periods of time, as shown by the dark red curve obtained after 1 hour at 280°C (Figure 2b). Overall, transmission electron microscopy reveals uniform, nearly spherical particles without clustering or aggregation (Figure 2c), confirming the stability and monodispersity of QDs synthesized and protected with multidentate ligands.
Figure 2.
Fluorescence emission and electron-microscopy structural properties of CdTe core QDs prepared by using multidentate polymer ligands in a one-pot procedure. (A) Color photograph of a series of monodispersed CdTe QDs, showing bright fluorescence from green to red (515 nm to 655 nm) upon illumination with a UV lamp. (B) Normalized fluorescence emission spectra of CdTe QDs with 35–50 nm full width at half maximum (FWHM). (C) Transmission electron micrograph of QDs showing uniform, nearly spherical particles.
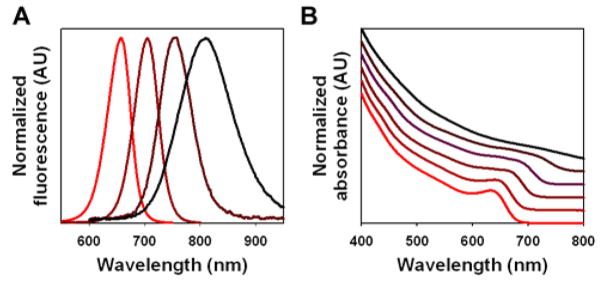
Because QD cores show a propensity to oxidize in water, we have developed an in-situ procedure for capping them with an inorganic passivating shell. Inorganic shells have the added benefit of increasing the quantum yield as well as opening the possibility of bandgap engineering through the selection of an appropriate shell material. In this procedure, an excess of cadmium is used to start the synthesis (the mole ratio of cadmium to tellurium mole is typically 2:1), and the reaction is allowed to proceed until the limiting species (tellurium) is depleted. This leaves an excess of the cadmium precursor available for incorporation into a passivating shell. CdSe is used as a model shell material for CdTe cores because the band offsets are such that CdTe/CdSe is a type-II QD with light emission in the near-infrared spectrum.11 Fluorescence emission spectra (Figure 3a) show significant red-shifting of the original QD core emission, from 650 nm to 810 nm, when a shell is grown on the particle surface. Considerable broadening of the emission peak is observed with shell growth, consistent with the behavior of type-II QDs. Monitoring of the QD absorbance spectra also confirms shell growth and transition to type-II behavior (Figure 3b). For example, the distinct exciton peak seen in the CdTe cores (red curve) is gradually red-shifted and eventually disappears during shell deposition. This is expected as the CdTe/CdSe QDs should behave as indirect semiconductors near the band edge.
Figure 3.
Type-II core-shell CdTe/CdSe QDs synthesized in one pot. (A) Normalized fluorescence emission spectra showing the transition from CdTe cores (red curve) to CdTe/CdSe core-shell QDs emitting in the near-infrared (black curve). (B) Optical absorbance showing the red-shifting and eventual loss of the first exciton peak as the CdSe shell is grown on the CdTe core, typical of type II QDs.
The roles of low-molecular weight PEGs are also interesting. They provide not only an inert and noncoordinating environment for QD synthesis at high temperatures, but also act as an “adjuvant” to facilitate nanocrystal dissolution in various solvents. Indeed, the QDs reported in this work show “amphibious” behaviors and are soluble in a wide range of hydrophilic and hydrophobic solvents including water, DMF, acetone, and chloroform. The detailed results on “amphibious nanocrystals” will be reported in a separate paper.
In summary, we have reported a new, one-pot procedure for preparing high-quality QDs based on the use of amphiphilic multidentate ligands and short polyethylene glycols at high temperatures. The novel features associated with the use of polymeric precursors are better control of the nanocrystals growth kinetics, resistance to Oswald ripening, and synthesis of ultrasmall dots with blue-shifted emission spectra. This synthetic procedure also allows for in-situ growth of an inorganic passivating shell (CdSe) on the QD core, opening the possibility of bandgap engineering for these nanoparticles and providing a large dynamic range for QD emission from the visible to the near infrared.
Acknowledgments
This work was supported by grants from the National Institutes of Health (P20 GM072069, R01 CA108468, U54CA119338, U01HL080711, and PNEY018244). B.A.K. acknowledges the NSF-IGERT program for stipend support; A.M.S. thanks the Whitaker Foundation for a graduate fellowship; and S.N. is a Distinguished Cancer Scholar of the Georgia Cancer Coalition (GCC).
Footnotes
Supporting Information Available: Detailed procedures for one-pot synthesis of core and core-shell QDs. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Schaller RD, Agranovich VM, Klimov VI. Nature Physics. 2005;1:189–194. [Google Scholar]; (b) Schaller RD, Klimov VI. Phys Rev Lett. 2004;92:186601. doi: 10.1103/PhysRevLett.92.186601. [DOI] [PubMed] [Google Scholar]
- 2.(a) Bruchez MP, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]; (b) Chan WCW, Nie SM. Science. 1998;281:2016. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]; (c) Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale F, Bruchez MP. Nat Biotechnol. 2003;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]; (d) Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. Science. 2002;298:1759–62. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 3.(a) Gao X, Cui Y, Levenson RM, Chung LW, Nie S. Nat Biotechnol. 2004;22:969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]; (b) Kim S, Lim YT, Soltesz EG, DeGrand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV. Nat Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CB, Norris DJ, Bawendi MG. J Am Chem Soc. 1993;115:8706–8715. [Google Scholar]
- 5.Peng ZA, Peng X. J Am Chem Soc. 2001;123:183–4. doi: 10.1021/ja003633m. [DOI] [PubMed] [Google Scholar]
- 6.Yu WW, Peng X. Angew Chem Int Ed. 2002;41:2368–71. doi: 10.1002/1521-3773(20020703)41:13<2368::AID-ANIE2368>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.(a) Hines MA, Guyot-Sionnest P. J Phys Chem. 1996;100(2):468–471. [Google Scholar]; (b) Dabbousi BO, RodriguezViejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, Jensen KF, Bawendi MG. J Phys Chem B. 1997;101:9463–9475. [Google Scholar]
- 8.(a) Gaponik N, Talapin DV, Rogach AL, Hoppe K, Shevchenko EV, Kornowski A, Eychmuller A, Weller H. J Phys Chem B. 2002;106:7177–7185. [Google Scholar]; (b) Zhang H, Wang LP, Xiong HM, Hu LH, Yang B, Li W. Adv Mat. 2003;15:1712–15. [Google Scholar]; (c) Li L, Qian HF, Ren JC. Chem Comm. 2005:528–530. doi: 10.1039/b412686f. [DOI] [PubMed] [Google Scholar]
- 9.(a) Qian HF, Qiu X, Li L, Ren JC. J Phys Chem B. 2006;110:9034–9040. doi: 10.1021/jp0539324. [DOI] [PubMed] [Google Scholar]; (b) He Y, Lu HT, Sai LM, Lai WY, Fan QL, Wang LH, Huang W. J Phys Chem B. 2006;110:13352–13356. doi: 10.1021/jp061719h. [DOI] [PubMed] [Google Scholar]; (c) He Y, Lu HT, Sai LM, Lai WY, Fan QL, Wang LH, Huang W. J Phys Chem B. 2006;110:13370–13374. doi: 10.1021/jp057498h. [DOI] [PubMed] [Google Scholar]
- 10.Celebi S, Erdamar AK, Sennaroglu A, Kurt A, Acar HY. J Phys Chem B. 2007;111:12668–12675. doi: 10.1021/jp0739420. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Fisher B, Eisler HJ, Bawendi M. J Am Chem Soc. 2003;125:11466–11467. doi: 10.1021/ja0361749. [DOI] [PubMed] [Google Scholar]