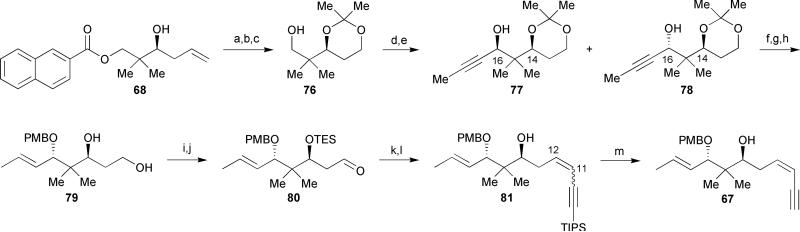
Scheme 11.
Wipf's enyne segment synthesis. Reagents and conditions: (a) O3/O2, Sudan III, MeOH/CH2Cl2, −78 °C, then NaBH4, −78 °C to rt, 88%; (b) 2,2-dimethoxypropane, PPTS, THF, 0 °C to rt, 97%; (c) 1N LiOH, THF/MeOH, 0 °C to rt, 82%; (d) oxalyl chloride, DMSO, Et3N, −78 °C; (e) propyne, n-BuLi, THF, −78 °C to 0 °C; (f) Red-Al, THF (degassed), Δ, 83%; (g) PMBBr, Et3N, KHMDS, THF, −78 °C to rt; (h) AcOH/THF/H2O (4:1:1), 60 °C, 84% (over 2 steps); (i) TESOTf, 2,6-lutidine, CH2Cl2, 0 °C; (j) oxalyl chloride, DMSO, Et3N, CH2Cl2, −78 °C, 75% (over 2 steps); (k) 1,3-bis(TIPS) propyne, n-BuLi, THF, −78 °C; (l) chloroacetic acid, MeOH/CH2Cl2; (m) TBAF, THF, 0 °C to rt, 94%.