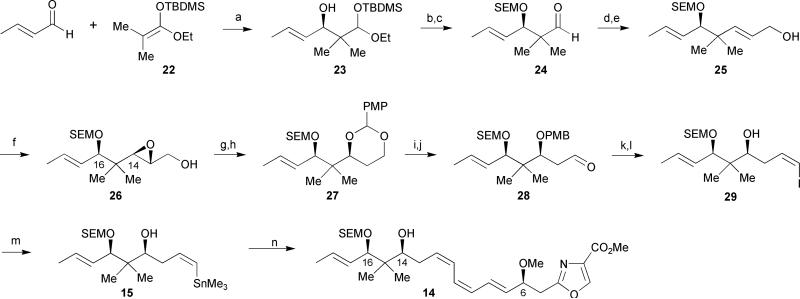
Scheme 2.
Meyers’ 1st generation synthesis of the southern fragment of disorazole C1. Reagents and conditions: (a) BH3·THF, N-Ts-L-Val, CH2Cl2, −78 °C, 73%; (b) SEMCl, Hünig's base, CH2Cl2, −78 °C; (c) 80% AcOH, 79% (over 2 steps); (d) (EtO)2P(O)CH2CO2Et, NaH, toluene/THF, 95%; (e) DIBAL-H, CH2Cl2, −78 °C, 76%; (f) D-(−)-DIPT, t-BuOOH, Ti(Oi-Pr)4, CH2Cl2, −30 °C, 95%; (g) Red-Al, THF, −20 °C: (h) p-methoxybenzylidene dimethyl acetal, PPTS, CH2Cl2, 83% (over 2 steps); (i) DIBAL-H, CH2Cl2, −78 °C, 92%; (j) Dess–Martin periodinane, pyridine, t-BuOH, CH2Cl2, 83%; (k) I−Ph3P+CH2I, NaHMDS, HMPA, THF, −78 °C, 67%; (l) DDQ, CH2Cl2, H2O, 79%; (m) PdCl2(PPh3)2, (Me3Sn)2, Li2CO3, THF, 74%; (n) PdCl2(CH3CN)2, 16, DMF, 76%.