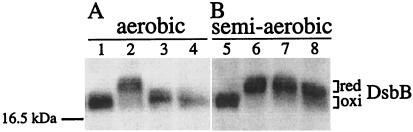
Figure 4.
Effect of oxygen limitation on the redox state of mutant DsbB. Strains HK205 (wild type; lanes 1 and 5), HK207 (dsbB C41Y; lanes 2 and 6), HK209 (dsbB R48C; lanes 3 and 7), and HK211 (dsbB R48H; lanes 4 and 8) were grown in a test tube aerobically in 5 ml (A) or semiaerobically in 20 ml (B) of M63 minimal medium. Proteins of exponentially growing cells were AMS-alkylated, separated by native SDS/PAGE, and subjected to Western blotting with anti-DsbB antibody to detect the oxidized (oxi) and the reduced (red) forms of DsbB. The position of a molecular mass standard is indicated on the left. Note that wild-type DsbB contains two additional nonessential cysteine residues that remain in the sulfhydryl state and are subject to alkylation with AMS. As a reference, the previously isolated dsbB C41Y mutant, which renders DsbB fully reduced, was included in lanes 2 and 6. The increase in the apparent molecular mass, as compared with the wild-type strain, agrees well with the difference in the number of free cysteine residues; 2 for wild-type and 5 for the C41Y mutant enzyme. Note also the difference in the migration of DsbBs. R48C has one additional cysteine over the wild type and R48H, thus it migrates slower than they do.