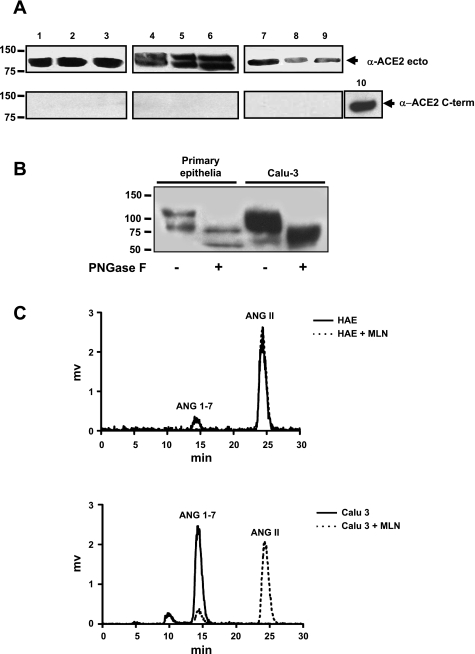
Fig. 1.
Soluble angiotensin-converting enzyme 2 (sACE2) protein is detected in apical secretions of polarized epithelial cells and in human bronchoalveolar lavage fluid (BAL) and is enzymatically active. A, top: immunoblot for sACE2 using antibody that recognizes the extracellular portion of protein. Samples include secretions from Calu-3 cells (lanes 1–3), primary epithelia (3 different donor samples; lanes 4–6), and bronchoalveolar lavage (3 different donor samples; lanes 7, 8, and 9). When loaded on SDS-PAGE gel for electrophoresis, Calu-3 cell washes were diluted 10-fold in PBS, and BAL samples were concentrated 10-fold. A, bottom: immunoblot for ACE2 using an antibody against a C-terminal protein epitope. Lane 10 is a positive control consisting of cell lysate from 293 cells transfected with ACE2 expression plasmid. B: sACE2 recovered from primary airway epithelia or Calu-3 cells was deglycosylated with N-glycanase PNGase F; bands of ∼120 kD were reduced to ∼85 kDa. C: chromatograms from sACE2 enzymatic activity assay. Surface washes from human airway epithelia (HAE) or Calu-3 cells converted ANG II to ANG-(1-7). Addition of the ACE2-specific antagonist MLN into the reaction markedly inhibited the conversion.