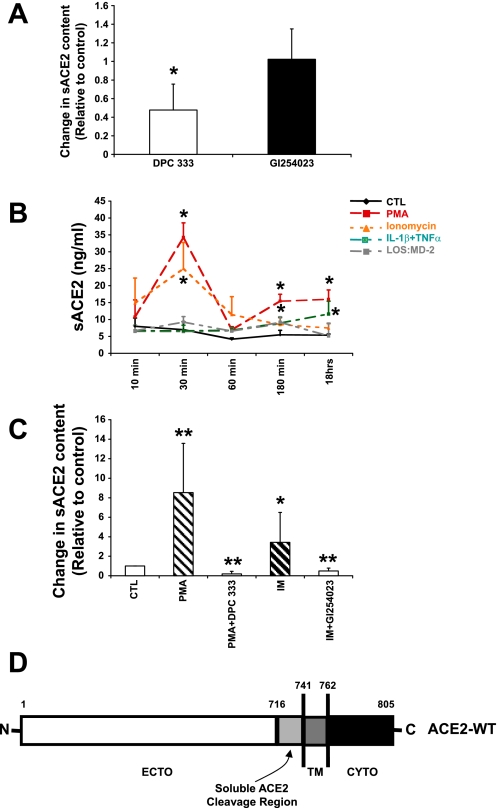
Fig. 3.
Induction of sACE2 release from air-liquid interface cultures of airway epithelia. A: a disintegrin and metalloprotease 17 (ADAM17) is responsible for constitutive ACE2 shedding in primary human airway epithelial cells. The ADAM17 inhibitor DPC 333 at 1.5 nM concentration inhibited about 50% ACE2 shedding in airway epithelia (P < 0.05, compared with control group; means ± SE; n = 3). The ADAM10 inhibitor GI254023 (5 μM) did not significantly change constitutive ACE2 shedding. B: induction of sACE2 release from primary epithelia. Indicated stimuli were added to either the apical surface or both apical and basolateral sides of polarized cells and apical solution was collected at the indicated experimental time points for ACE2 ELISA assay. LOS:MD-2 is a TLR4 ligand. Data are means ± SE from 3 human donor samples. *P < 0.05, compared with the control group by Student's t-test. C: ADAM17 inhibitor DPC 333 (1.5 nM) blocks PMA-stimulated sACE2 release; the ADAM10 inhibitor GI254023 (5 μM) blocks ionomycin (IM) stimulated sACE2 release. Primary epithelia were treated with indicated conditions for 60 min and sACE2 recovered from apical surface and abundance determined by ELISA. Data are means ± SE from 6 human donor samples. *P < 0.05 and **P < 0.01, compared with the control group by Student's t-test. D: ACE2 cleavage site. Diagrammatic representation of protein sequence data. The identity of the C-terminal most peptide fragment indicates region where cleavage occurs.