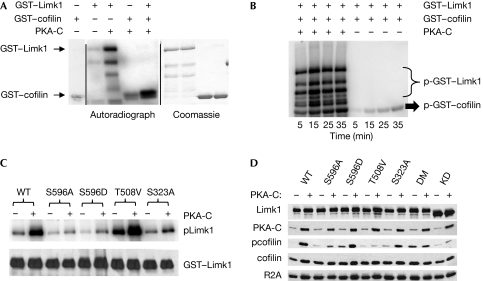
Figure 3.
PKA phosphorylates Limk1 and increases its ability to phosphorylate cofilin. (A) GST–Limk1 and GST–cofilin were subject to an in vitro kinase assay with PKA-C. The left panel shows the autoradiograph, whereas the right panel shows total protein lysates stained with Coomassie. The panel at the far left shows GST–cofilin incubated without other proteins to assess the background level of the assay. (B) Autoradiograph of the time course of Limk1-mediated phosphorylation of cofilin with or without pre-incubation with PKA-C. (C) Autoradiograph of purified WT or phosphomutant Limk1 proteins treated with PKA-C in vitro. Note reductions in the label caused by mutation of Ser 596 or Ser 323 but not of Thr 508. (D) Immunoblot analysis of lysates from 293T cells transfected with WT or phosphomutant Limk1 with and without PKA-C. DM indicates S596A/S323A double mutant, whereas KD indicates the D460A dominant-negative (kinase-dead) mutant. Prkar2a (R2A) is shown as a loading control. GST, glutathione S-transferase; PKA, protein kinase A; WT, wild type.