Figure 1.
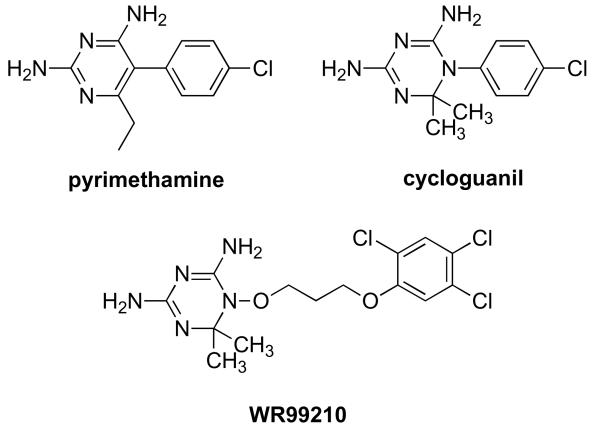
Figure 1a Structure of WR99210 compared to those of clinically used antifolates, pyrimethamine and cycloguanil.
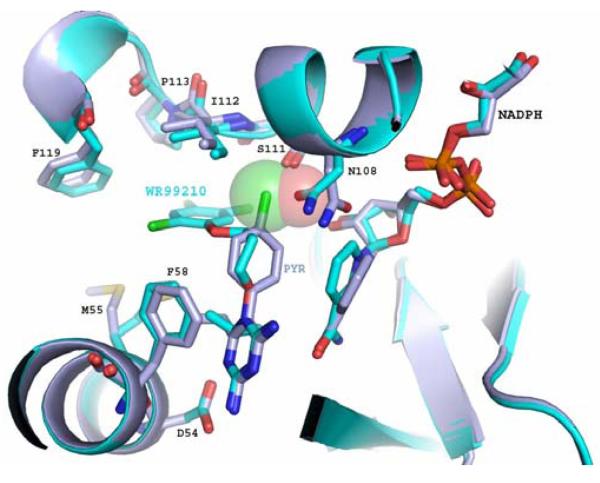
Figure 1b WR99210 (WR, cyan) binds successfully to the active site of the clinically important, drug-resistant, N51I/C59R/S108N/I164L “quadruple” mutant of P. falciparum DHFR. Unlike Pyrimethamine (Pyr, purple), WR99210 avoids steric hindrance with Asn108, which clashes with the p-chlorophenyl ring of pyrimethamine, the DHFR inhibitor in Fansidar™. NADPH, an essential cofactor in the conversion of H2-folate to H4-folate by DHFR, is shown bound to the DHFR active site as well.