Figure 1.
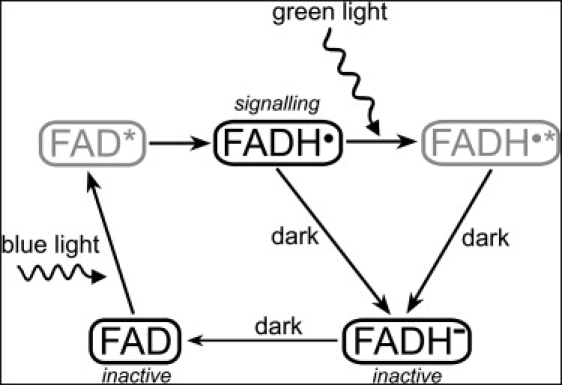
Light-induced photocycle in cryptochrome. The signaling state of cryptochrome is controlled by the oxidation state of its flavin cofactor FAD, which exists in three interconvertible redox forms, FAD, FADH•, and FADH– (56,60,61). The FAD form is inactive (nonsignaling) and accumulates to high levels in the dark. Blue light triggers photoreduction of FAD to establish a photoequilibrium that favors FADH• over FAD or FADH–. The semiquinone FADH• state corresponds to the signaling state of the protein. Green light photons can further be absorbed by the radical FADH• and shift the photoequilibrium to the fully reduced form (FADH–), which is inactive (nonsignaling). The FAD → FADH• and FADH• → FADH– reactions involve an active FADH• radical and, therefore, can be affected by an external magnetic field. The excited states of the flavin cofactor, FAD∗ and FADH•∗, colored gray, arise as short-lived intermediate stages of the cryptochrome photocycle.
