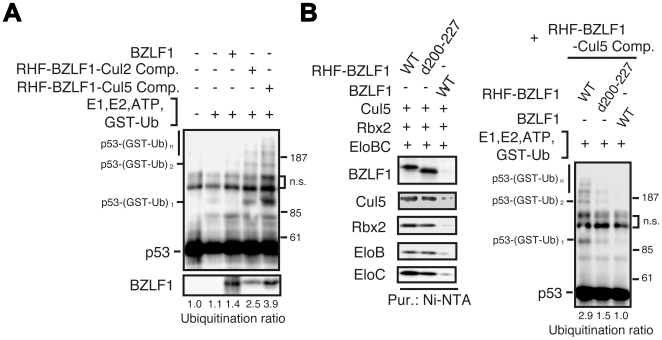
Figure 2. Ubiquitination of p53 by recombinant BZLF1-ECS complexes in vitro.
(A) Recombinant BZLF1 protein, and BZLF1-EC2S or BZLF1-EC5S complexes were assayed for their ability to mediate the ubiquitination of p53 in the presence of ATP, Uba1 (E1), UbcH5c (E2) and GST-Ub. Reaction mixtures were incubated for 1 h at 26°C, boiled in SDS sample buffer, and then subjected to IB analysis with anti-p53 antibody. Intensity of the polyubiquitin chains is expressed as a ratio between polyubiquitinated p53 protein and p53 protein input. (B) The BZLF1 protein acts as an adaptor protein to recognize the substrate for p53 ubiquitination. Wild type BZLF1 (WT) or d200-227 mutant protein was expressed with components of the EC5S complex in Sf21 cells, and cell lysates were subjected to Ni-NTA affinity purification. The purified BZLF1 complexes were applied to the reaction and then IB analysis with anti-p53 antibody (right panels). Each recombinant BZLF1-EC5S complex purified from Sf21 insect cell lysates was analyzed by IB with the indicated antibodies (left panels).