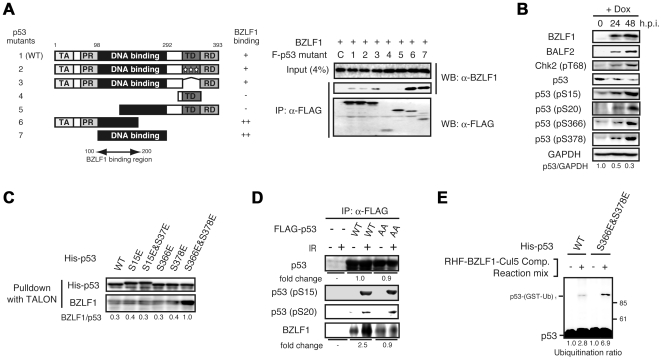
Figure 3. Phosphorylation-dependent enhancement of the interaction between p53 and BZLF1 protein.
(A) BZLF1 protein interacts with the DNA-binding domain near the N-terminal of p53. SaOS-2 cells were cotransfected with BZLF1 protein and a series of FLAG-tagged p53 deletion mutant expression plasmids as indicated. Cell lysates were prepared 24 h post-transfection, and equal amounts of proteins under each condition were incubated with anti-FLAG M2 antibody beads. Immunoprecipitated proteins were analyzed by immunoblotting with anti-BZLF1 protein antibodies. Schematic illustration of the p53 wild-type and deletion mutants used. TA, transactivation domain; PR, proline rich domain; TD, tetramerization domain; RD, regulatory domain. (B) p53 is hyperphosphorylated during lytic infection. Tet-BZLF1/B95-8 cells were cultured with Dox for the indicated times. For p53 phosphorylation at S366 and S378 residues, IP/IB analysis was performed, while for the others IB analysis with the indicated specific antibodies was carried out. The p53/GAPDH ratio is provided. (C) Interaction of the BZLF1 protein with WT p53 and a variety of phospho-mimetic mutants. His-tagged p53 proteins were first attached to TALON beads and then incubated with purified recombinant BZLF1 protein in PBS. The beads were washed and spun down. Bound BZLF1 protein was detected by IB with anti-BZLF1 antibody. The intensity of the bands is expressed as a ratio between BZLF1 protein and p53. (D) The requirement of the p53 phosphorylation at C-terminus for the enhancement of binding to BZLF1 protein. 293T cells were transfected with FLAG-p53 (WT or S366A&S378A) expression vector, exposed to ionizing radiation (20 Gy) 46 h post-transfection and harvested 48 h post-transfection. FLAG-tagged p53 proteins were first purified using anti-FLAG antibody resin from cell extracts, and then incubated with purified recombinant BZLF1 protein. The beads were washed and spun down. Bound protein was detected by IB with indicated antibodies. AA indicates p53 S366A&S378A mutant. The band intensity was quantified as a fold change of IR-treated/untreated. (E) The phospho-mimic p53 mutant is more ubiquitinated than wild-type p53 by BZLF1- EC5S complex in vitro. Recombinant His-p53 protein (WT or S366E&S378E) was incubated in a reaction mixture containing purified BZLF1-EC5S complex. Reactions for p53 ubiquitination were carried out as described in the legend for Figure 2. The ubiquitinated p53/p53 ratio is indicated.