Figure 3.
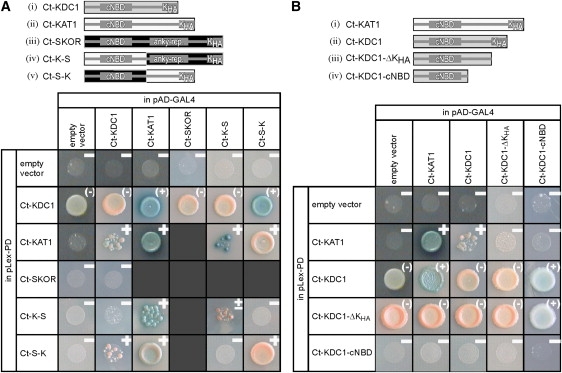
Yeast two-hybrid studies on the interaction between the C-termini of potassium channels. Interactions were monitored in a drop test on Leu--Trp--His- medium containing 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside. Blue dye formation and, in part, growth report the physical association of the coexpressed fusion proteins. A positive answer is denoted by a ‘‘+’’, a negative answer by a ‘‘−’’. The presented results are representatives of at least three independent experiments each. Note that the fusions of Ct-KDC1 and Ct-KDC1-ΔKHA to the LexA binding domain enable yeast to grow on histidine-free medium even in the absence of an interacting protein (Ct-KDC1 + empty vector, Ct-KDC1-ΔKHA + empty vector). In these cases, HIS3 is not a suitable reporter gene for protein-protein interactions, and therefore only the blue dye formation was interpreted as a positive answer (indicated as (+)). (A) Identification of intermolecular interactions between the C-termini of KAT1, SKOR, and KDC1. A schematic illustration of the tested C-termini and chimeras is shown in the upper panel. Parts originating from KDC1 are illustrated by gray rectangles, parts from KAT1 by white rectangles, and parts from SKOR are shown as black rectangles. The C-terminal regions Ct-KDC1 (=KDC1-A310_F629) (i), Ct-SKOR (=SKOR-D338_T828) (ii), Ct-KAT1 (=KAT1-T308_N677) (iii), Ct-K-S (=KAT1-T308_D544-SKOR-P574_T828) (iv), Ct-S-K (=SKOR-D338_D573-KAT1-T545_N677) (v) were fused to the LexA DNA binding domain of the vector pLexPD and to the GAL4 activator domain of the vector pAD-GAL4. The interactions between Ct-SKOR and Ct-KAT1, Ct-K-S, and Ct-S-K were tested and reported earlier (11). (B) Identification of structural domains of KDC1 being responsible for the interaction with KAT1. A schematic illustration of the tested full-length and truncated C-termini is shown in the upper panel. Parts originating from KDC1 are illustrated by gray rectangles, parts from KAT1 by white rectangles: the C-terminal regions Ct-KAT1 (=KAT1-T308_N677) (i), Ct-KDC1 (=KDC1-A310_F629) (ii), Ct-KDC1-ΔKHA (=KDC1-A310_T578) (iii), and Ct-KDC1-cNBD (=KDC1-A310_F495) (iv) were fused to the LexA DNA binding domain of the vector pLexPD and to the GAL4 activator domain of the vector pAD-GAL4.
