Abstract
Background and Aims
The metabolism of β-1,3 : 1,4-glucan regulates the mechanical properties of cell walls, and thereby changes the elongation growth of Poaceae plants. A previous study has shown that elongation growth of rice coleoptiles under water is enhanced by increased activity of β-1,3 : 1,4-glucan hydrolases; however, the involvement of β-1,3 : 1,4-glucan synthase activity in elongation growth under water has not yet been clarified.
Methods
The β-1,3 : 1,4-glucan synthase activity in a microsomal fraction prepared from rice seedlings grown under water was compared with that from control seedlings grown in air. The change under water in the relative expression level of CslF6, a major isoform of the β-1,3 : 1,4-glucan synthase genes, was examined by quantitative reverse-transcriptase PCR.
Key Results
The level of β-1,3 : 1,4-glucan synthase activity in submerged seedlings decreased to less than 40 % of that of the control seedlings and was accompanied by a significant reduction in the amount of β-1,3 : 1,4-glucan in the cell walls. Under water, the expression of CslF6 was reduced to less than 20 % of the unsubmerged control. Bubble aeration partially restored both β-1,3 : 1,4-glucan synthase activity and the expression of CslF6 under water, correlating with suppression of the submergence-induced elongation growth of coleoptiles.
Conclusions
Submergence down-regulates the expression of the CslF6 gene, leading to a decreased level of β-1,3 : 1,4-glucan synthase activity. Together with the increased activity of β-1,3 : 1,4-glucan hydrolases, the decreased activity of β-1,3 : 1,4-glucan synthase contributes to the decrease in the amount of β-1,3 : 1,4-glucan in the cell walls under water. The suppression of β-1,3 : 1,4-glucan synthesis under water may be mainly due to oxygen depletion.
Key words: β-1,3 : 1,4-glucan; rice; Oryza sativa; elongation growth; cell wall; β-1,3 : 1,4-glucan synthase; CslF gene
INTRODUCTION
The high molecular mass polysaccharide β-1,3 : 1,4-glucan is a cell wall component that is widely distributed in Poaceae plants such as rice, barley and maize. It consists primarily of cellotriosyl and cellotetraosyl units linked by single β-1,3-glucosidic linkages (Carpita and Gibeaut, 1993; note that sugars in this article belong to the D-series unless indicated otherwise). This polysaccharide also contains a small portion of long stretches of β-1,4-linked glucosyl residues (Kato and Nevins, 1984), which enables the glucan to interact with other β-1,3 : 1,4-glucan, arabinoxylan and cellulose microfibrils by hydrogen bonding. Several lines of evidence indicate that the metabolism of β-1,3 : 1,4-glucan is associated substantially with the cell wall loosening responsible for the elongation growth of Poaceae plants. For example, antibodies against β-1,3 : 1,4-glucan suppress the auxin-induced elongation growth of Avena coleoptiles (Hoson and Nevins, 1989). Perturbation of the degradation of β-1,3 : 1,4-glucan with antibodies raised against β-1,3 : 1,4-glucan hydrolases significantly inhibits auxin-induced elongation growth of maize coleoptiles (Inouhe and Nevins, 1991b). It has been shown that Poaceae plants possess endo-β-1,3 : 1,4-glucanase (EC 3·2·1·73), endo-β-1,4-glucanase (EC 3·2·1·4) and exo-β-glucanase (EC 3·2·1·58) that catalyse the degradation of β-1,3 : 1,4-glucan (Fincher et al., 1986; Hrmova et al., 1996; Kotake et al., 2000; Yoshida and Komae, 2006). Although the contribution of the degradation of β-1,3 : 1,4-glucan by β-1,3 : 1,4-glucan hydrolases to elongation growth has been established in Avena (Loescher and Nevins, 1972), maize (Inouhe and Nevins, 1991a, b), rice (Zarra and Masuda, 1979a, b) and barley (Sakurai and Masuda, 1978; Kotake et al., 2000), the impact of the synthesis of β-1,3 : 1,4-glucan on the elongation growth of Poaceae plants is so far unknown.
Higher plants exhibit a variety of physiological reactions in response to submergence. Alcoholic fermentation, through which pyruvate is converted to acetaldehyde by the action of pyruvate decarboxylase (EC 4·1·1·1) and then to ethanol by alcohol dehydrogenase (ADH, EC 1·1·1·1), enables plants to generate ATP by recycling NAD+ under low oxygen conditions. The importance of alcoholic fermentation for elongation growth of rice coleoptiles under anaerobic conditions has been clearly demonstrated by studies on the rice adh mutant with a defect in alcohol dehydrogenase (Meguro et al., 2006; Saika et al., 2006). Induction of sucrose synthase (SuSy, EC 2·4·1·13) is also a significant reaction induced under anaerobic conditions (McElfresh and Chourey, 1988; Ricard et al., 1991). SuSy is presumed to play an important role in the generation of fermentable hexoses from sucrose, and thus to contribute to the generation of ATP through glycolysis. It is highly probable that synthesis of cell wall polysaccharides also affects the levels of fermentable sugars under anaerobic conditions, since nucleotide sugars such as UDP-glucose (UDP-Glc) are consumed during the syntheses of cell wall polysaccharides. However, the effect of submergence on the synthesis of the polysaccharides has not been examined because it is difficult to measure the activities of the corresponding glycosyltransferases.
Biosynthesis of β-1,3 : 1,4-glucan is catalysed by β-1,3 : 1,4-glucan synthase (no EC number: classified into glycosyl transferase family 2, http://www.cazy.org/) using UDP-Glc as the substrate. Microsomal fractions prepared from various Poaceae plants exhibit activity of β-1,3 : 1,4-glucan synthase (Becker et al., 1995; Tsuchiya et al., 2005). The enzyme transfers the Glc units from UDP-Glc to endogenous acceptors contained in microsomal fractions in vitro. In topographic experiments, the enzyme has been shown to localize at the Golgi apparatus and consumes substrate UDP-Glc at the cytosolic side of the Golgi membrane (Urbanowicz et al., 2004). Based on the chemical similarities between cellulose (β-1,4-glucan) and β-1,3 : 1,4-glucan, it has been proposed that β-1,3 : 1,4-glucan synthase is a member of the CesA-like (Csl) protein family. Recently, a group of cellulose synthase-like proteins, the CslF proteins exclusively distributed in Poaceae plants, was identified using bioinformatics (Hazen et al., 2002) and shown to participate in the synthesis of β-1,3 : 1,4-glucan by a heterologous expression experiment of the gene in Arabidopsis (Burton et al., 2006). The rice genome contains at least eight genes encoding CslFs, but their individual expression patterns and physiological functions are unknown. It seems likely that expression of the CslF genes involved in the synthesis of β-1,3 : 1,4-glucan is regulated by growth stage and environmental conditions.
A previous study has indicated that submergence increases the level of β-1,3 : 1,4-glucan hydrolase activity, leading to stimulation of elongation growth of rice coleoptiles (Chen et al., 1999a). For the present study, we examined the effect of submergence on β-1,3 : 1,4-glucan synthase activity in rice seedlings. The results indicate that the level of β-1,3 : 1,4-glucan synthase activity is strongly affected by submergence.
MATERIALS AND METHODS
Plant materials and growth conditions
Rice (Oryza sativa L. ‘Koshihikari’) caryopses were sterilized with sodium hypochlorite for 30 min, sown on 0·9 % (w/v) agar in a glass beaker (85 mm internal diameter, 115 mm height) and grown in the dark for 60 h at 26–28 °C. Submergence was achieved by adding 400 mL (depth 70 mm) of distilled water adjusted to 26–28 °C to the seedlings. After incubation under water for 12 h, the rice seedlings including coleoptiles and young leaves were harvested. Aeration experiments were performed with an air pump and a bubbling filter. For the control, the seedlings were grown for 72 h in air without addition of water.
Assay for β-1,3 : 1,4-glucan synthase activity
Microsomal fractions were prepared from rice seedlings as described by Becker et al. (1995) and Tsuchiya et al. (2005). Rice seedlings were homogenized with a mortar and pestle in 50 mm 2-amino-2-hydroxymethyl-1,3-propanediol (Tris)-HCl buffer (pH 8·5), 1 mm EGTA. The homogenate was centrifuged at 1200 g for 15 min to remove the precipitate. The supernatant was ultracentrifuged at 100 000 g for 60 min, and the resulting pellet containing microsomes was washed with the homogenation buffer and suspended in the buffer. The protein content was determined by the method of Bradford (1976) using bovine serum albumin as the standard.
We modified the method described by Becker et al. (1995) to measure the enzyme activity of β-1,3 : 1,4-glucan synthase. The reaction mixture (60 µL), consisting of 2 mm UDP-Glc [including 0·08 µCi UDP-(14C)Glc, Perkin Elmer Life Science Inc., Boston, MA, USA], 20 mm MgCl2, 200 mm sucrose, 50 mm Tris–HCl buffer (pH 9·0), and the microsomal fraction (approximately 400 µg protein) were incubated at 25 °C for 30 min. The reaction was terminated by dipping in a boiling water bath for 5 min. To precipitate the products thoroughly, 0·2 % β-1,3 : 1,4-glucan (36 µL) as a carrier and methanol (120 µL) were added to the reaction mixture. After standing on ice for 10 min, the insoluble materials containing the radio-labelled products were collected by centrifugation at 8000 g for 10 min, and twice washed with 70 % methanol (750 µL). The products in the precipitate were dissolved in 20 mm 3-morpholinopropanesulfonic acid (MOPS)-KOH buffer (pH 6·5, 90 µL) at 100 °C for 5 min, and digested into diagnostic oligosaccharides such as 3-O-cellobiosyl-glucose (G4G3G; here ‘G’ indicates β-D-glucopyranose, and ‘4’ and ‘3’ indicate β-1,4-glucosidic linkage between non-reducing terminal and penultimate glucoses, and β-1,3-glucosidic linkage between penultimate and reducing terminal glucoses, respectively) and 3-O-cellotriosyl-glucose (G4G4G3G) with 1 unit (10 µL) of lichenase (EC 3·2·1·73, 118 units mg–1 protein, Megazyme, Wicklow, Ireland) specific to β-1,3 : 1,4-glucan for 12 h at 32 °C. Very low levels of contamination of other enzymes such as α-amylase (less than 0·5 milliunits mg–1 protein) and endo-β-1,4-glucanase (less than 0·1 milliunit mg–1 protein) in the enzyme specimen allowed the specific digestion of β-1,3 : 1,4-glucan into the diagnostic oligosaccharides.
The oligosaccharides released were separated by paper chromatography using 6 : 4 : 3 (v/v/v) 1-butanol : pyridine : water as the solvent. The radioactive spots corresponding to the oligosaccharides were detected by a Bio-Image Analyzer (BAS-1000, Fuji Photofilm Co., Tokyo, Japan), and excised from the paper. The amount of β-1,3 : 1,4-glucan produced by the enzyme reaction was determined by measuring the radioactivity of the spots with a liquid scintillation counter.
Determination of amount of β-1,3 : 1,4-glucan in cell walls
Rice seedlings were homogenized with a mortar and pestle in water, and centrifuged at 100 g for 15 min to collect the cell walls as the precipitate. The cell walls were first washed with ice-cold water twice, and boiled in 80 % ethanol for 15 min. To remove amylose, the cell walls were treated with 100 units of α-amylase (EC 3·2·1·1, type VII-A, porcine pancreatic, Sigma-Aldrich, St. Louis, MO, USA) for 4 h at 37 °C and then centrifuged. After removal of the supernatant, the cell walls were treated three times with water (1 mL) for 15 min at 100 °C, and three times with 50 mm EDTA (1 mL) for 15 min at 100 °C to remove pectic substances. Hemicellulose, including β-1,3 : 1,4-glucan, was extracted twice with 17·5 % NaOH containing 0·04 % NaBH4 (1 mL) for 15 min at 100 °C. The hemicellulose was neutralized with glacial acetic acid (1·5 mL), and dialysed against water. To determine the amount of β-1,3 : 1,4-glucan included in the cell walls, the hemicellulose was digested with lichenase (10 units) for 48 h at 37 °C. The released oligosaccharides derived from β-1,3 : 1,4-glucan were subjected to high-performance anion-exchange chromatography with pulsed amperometry detection (HPAEC-PAD) using a Dionex DX-500 liquid chromatograph (Dionex, Osaka, Japan) fitted with a CarboPac PA-1 column (4 × 150 mm) and a pulsed amperometric detector as described previously (Ishikawa et al., 2000). The amount of β-1,3 : 1,4-glucan was estimated based on the peak area of diagnostic oligosaccharides on HPAEC-PAD using G4G4G3G and G4G3G prepared from barley β-1,3 : 1,4-glucan from Megazyme as the standard.
Measurement of relative expression level of CslFs
Rice seedlings grown in air or under water were frozen in liquid nitrogen and homogenized with a mortar and pestle. Total RNA was extracted with an Isogen kit (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. Single-strand cDNA was synthesized from approximately 1 µg of total RNA from the seedlings using a reverse-transcriptase (RT), ReverTra Ace-α- (Toyobo, Osaka, Japan) and oligo(dT12–18) primer (Invitrogen, Carlsbad, CA, USA). A set of degenerate primers, CslF-dF (5′-TCSCTNGAGATSTTCTTCTC-3′) and CslF-dR (5′-CATSHAGAACTGCYCRTTSC-3′) was designed in the regions conserved for CslF genes based on the rice genome database (http://riceblast.dna.affrc.go.jp/). The PCR was performed with the set of degenerate primers using the single-strand cDNA as a template under the following conditions: 0·5 min denaturing at 95 °C, 0·5 min annealing at 55 °C and 1·5 min amplification at 72 °C, 35 cycles. The amplified cDNA fragment encoding an approx. 0·3-kb region of CslF genes was subcloned into a pGEM T-Easy vector (Promega, Madison, WI, USA) and the nucleotide sequences of the clones were determined with an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
The relative amount of CslF6 mRNA was estimated by quantitative PCR. A set of specific primers for the CslF6 gene (Os08g06380), CslF6-RTP-F (5′-CGCTACTGCTCCATCTACCC-3′) and CslF6-RTP-R (5′- GGCACGGTGGTGTAGAAGAT-3′), and for ACTIN1 (ACT1), ACT1-RTP-F1 (5′-TTCCTACATCGCCCTGGACT-3′) and ACT1-RTP-R1 (5′-AGCCTTGGCAATCCACATCT-3′) were designed using the Primer 3·0 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The PCR was performed with a SYBR Premix ExTaq kit (Takara Bio Inc., Otsu, Japan) under the following conditions: 10 sec denaturing at 95 °C, 30 sec annealing at 60 °C, and 20 sec amplification at 72 °C, 40 cycles. The PCR product was detected with Opticon 2 (Bio-Rad, Hercules, CA, USA) and the relative amount of CslF6 mRNA to ACT1 mRNA was calculated.
RESULTS
Activity of β-1,3 : 1,4-glucan synthase in rice seedlings
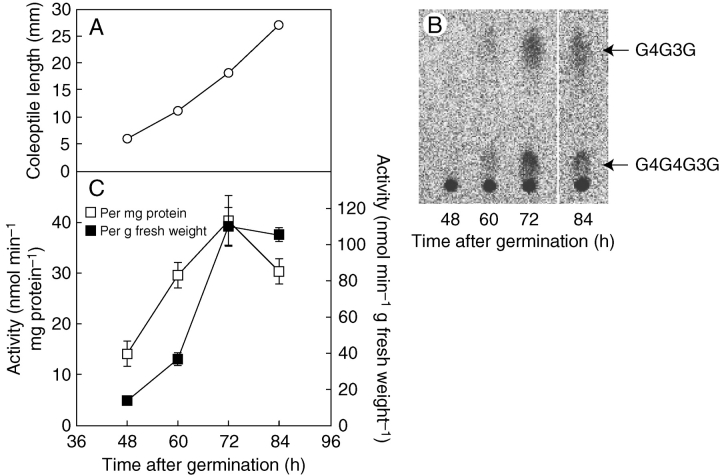
β-1,3 : 1,4-glucan synthase activity was first examined in rice seedlings between 48 and 84 h (2·0 and 3·5 d) after germination in air in the dark, since active elongation growth of the coleoptiles was observed during this growth stage (Fig. 1A). Using 14C-labelled UDP-Glc as the substrate, a microsomal fraction prepared from the seedlings catalysed the synthesis of β-1,3 : 1,4-glucan, which was degraded into diagnostic oligosaccharides such as G4G3G and G4G4G3G by digestion with lichenase (Fig. 1B). The results confirmed the presence of β-1,3 : 1,4-glucan synthase in the seedlings. The level of β-1,3 : 1,4-glucan synthase in the seedlings rose dramatically at 3 d and reached its maximum (Fig. 1C). Hence, seedlings grown for 60 h (2·5 d) in air were submerged for 12 h, and the effect on the level of β-1,3 : 1,4-glucan synthase activity was examined.
Fig. 1.
Detection of β-1,3 : 1,4-glucan synthase activity in rice seedlings grown in air. (A) Length of rice coleoptiles at various growth stages. (B) β-1,3 : 1,4-glucan synthase activity in microsomes prepared from the rice seedlings grown in air was assayed using a reaction mixture containing UDP-[14C]Glc as the substrate. The diagnostic oligosaccharides G4G3G and G4G4G3G are indicated, released from the β-1,3 : 1,4-glucan product by the action of lichenase followed by paper chromatographic separation. (C) β-1,3 : 1,4-glucan synthase activities per mg protein and per g fresh weight, determined based on the radioactivity of G4G3G and G4G4G3G. Values are means ± s.e of triplicate assays.
Effect of submergence on activity of β-1,3 : 1,4-glucan synthase
In a previous study, stimulation of elongation growth had been observed in coleoptiles of rice seedlings germinated under water (Chen et al., 1999a). A similar stimulation of elongation growth was observed as a consequence of a temporary treatment of submergence for 12 h in the present study (Fig. 2A). Submergence reduced the level of β-1,3 : 1,4-glucan synthase activity to less than 40 % of that of control seedlings (Fig. 2B). The decrease in β-1,3 : 1,4-glucan synthase activity was observed on the basis of per mg protein as well as on per g fresh weight. The increased elongation growth under water was almost completely suppressed by aeration, suggesting that hypoxia was the main cause of elongation growth stimulation. The level of β-1,3 : 1,4-glucan synthase activity (per mg protein) under water was restored from 32 % to 78 % of that of control seedlings by aeration. The reduced β-1,3 : 1,4-glucan synthase activity seems to decelerate the deposition of newly synthesized β-1,3 : 1,4-glucan with high molecular mass in the cell walls, which probably leads to change in the mechanical properties of the walls of the coleoptiles under water.
Fig. 2.
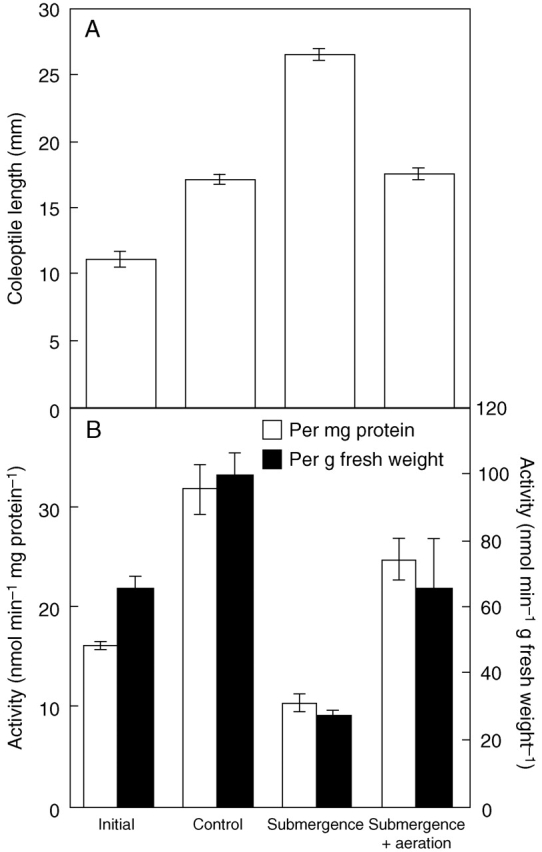
Effects of submergence on β-1,3 : 1,4-glucan synthase activity. (A) Length of coleoptiles before the submergence treatment (initial), grown for 12 h under water without aeration (submergence), with aeration (submergence + aeration) and in air (control). (B) β-1,3 : 1,4-glucan synthase activities per mg protein and per g fresh weight in the seedlings. Values are means ± s.e. of triplicate assays.
Change in amount of β-1,3 : 1,4-glucan
To confirm the suggested change in the metabolic outcome of β-1,3 : 1,4-glucan synthase activity under water, the amount of β-1,3 : 1,4-glucan in the cell walls was examined. The 12-h submergence treatment significantly reduced the amount of β-1,3 : 1,4-glucan in the seedlings (Table 1). The results indicate that the decrease in β-1,3 : 1,4-glucan synthase activity is concomitant with the decrease in the amount of β-1,3 : 1,4-glucan in the seedlings under water. However, we cannot exclude the possibility that the decreased amount of β-1,3 : 1,4-glucan is caused mainly by the acceleration of degradation catalysed by β-1,3 : 1,4-glucan hydrolases.
Table 1.
Effect of submergence on the level of β-1,3 : 1,4-glucan in tissues
| Amount in tissues (mg g−1 f. wt)* | Relative amount (%)† | |
|---|---|---|
| Initial | 1·51 ± 0·11 | 100·0 |
| Control | 1·49 ± 0·02 | 98·7 |
| Submergence‡ | 1·11 ± 0·05 | 73·5 |
* β-1,3 : 1,4-glucan extracted from rice seedlings was degraded into β-1,3 : 1,4-glucan-specific oligosaccharides. The level of β-1,3 : 1,4-glucan was determined based on the amount of the oligosaccharides using an HPAEC-PAD system. Data are means ± s.e. of triplicate assays.
† Relative amounts are expressed as a percentage of that of initial seedlings.
‡ Rice seedlings were grown for 12 h under water.
Down-regulation of expression of CslF6 under water
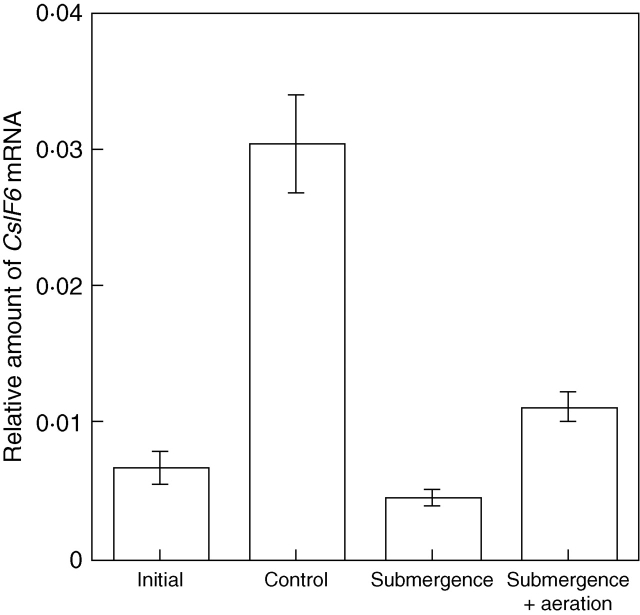
CslFs are a variety of Csl protein found exclusively in Poaceae plants and have been shown to be required for the synthesis of β-1,3 : 1,4-glucan. Rice possesses at least eight CslF genes designated CslF1, 2, 3, 4, 6, 7, 8 and 9, together with one pseudogene, CslF5 (Hazen et al., 2002; Burton et al., 2006). To identify CslF species expressed in rice seedlings, RT-PCR was first performed with a set of degenerate primers designed in the regions highly conserved for the CslF genes. Out of 14 clones isolated by RT-PCR, ten encoded CslF6, two CslF8, and two CslD2, indicating that CslF6 is at least one of the major CslF isoforms expressed in the seedlings. Hence, the change in the expression level of CslF6 under water was examined by quantitative RT-PCR. Under water, the expression level of CslF6 relative to ACT1 in the seedlings decreased to less than 20 % of that in the control seedlings (Fig. 3). As observed for the activity of β-1,3 : 1,4-glucan synthase, the expression level of CslF6 under water was restored by aeration. However, the recovery of CslF6 expression from 15 % to 36 % of the control seedlings was insufficient to explain the substantial restoration of the β-1,3 : 1,4-glucan synthase activity (from 32 % to 78 % of the control seedlings, if compared based on activity per mg protein) under water by aeration (Fig. 2B).
Fig. 3.
Change in relative amount of CslF6 mRNA under water. The relative amount of CslF6 mRNA to ACT1 mRNAs in the seedlings was measured by quantitative RT-PCR. Treatments are as described in Fig. 2. Values are means ± s.e. of triplicate assays.
DISCUSSION
The amount and molecular mass of cell wall polysaccharides are regulated by both degradation catalysed by glycoside hydrolases and synthesis by glycosyltransferases. Our previous studies have indicated that environmental stimuli such as temperature (Nakamura et al., 2003), light (Chen et al., 1999b) and microgravity (Hoson et al., 2002) affect the elongation growth of rice coleoptiles through changes in the level of β-1,3 : 1,4-glucan hydrolases in the cell walls. Submergence has been shown to raise the level of β-1,3 : 1,4-glucan hydrolases in rice coleoptiles, causing stimulation of elongation growth (Chen et al., 1999a). However, the involvement of β-1,3 : 1,4-glucan synthase activity in elongation growth has not been studied so far. In the present study, the level of β-1,3 : 1,4-glucan synthase activity in rice seedlings was found to decrease under water along with the amount of β-1,3 : 1,4-glucan in the cell walls (Table 1, Fig. 2). Based on these results, we propose that stimulation of the elongation growth of rice coleoptiles under water is mediated by a decrease in the level of β-1,3 : 1,4-glucan synthase activity together with an increase in the level of β-1,3 : 1,4-glucan hydrolase activity (Chen et al., 1999a). This is the first study to indicate the possibility that the level of β-1,3 : 1,4-glucan synthase activity changes in response to environmental stimuli in order to regulate elongation growth in Poaceae plants. Quantitative RT-PCR indicated that the expression level of CslF6 under water was less than 20 % of that in control seedlings (Fig. 3). This drastic change in the expression level of CslF6 probably alters the β-1,3 : 1,4-glucan metabolism and thereby causes at least partially the stimulation of the elongation growth of rice coleoptiles under water. In the present study, we could not divide the seedlings into coleoptiles and leaves and measure enzyme activity separately, because of the technical difficulty that β-1,3 : 1,4-glucan synthase activity is not stable and may be affected by procedures to isolate the coleoptiles. Therefore, we cannot exclude the possibility that the decreased activity of β-1,3 : 1,4-glucan synthase and the down-regulation of CslF6 expression occur in the young leaf of rice seedlings rather than in the coleoptiles under water. In a future study, we plan to examine β-1,3 : 1,4-glucan synthase for isolated coleoptiles.
The level of β-1,3 : 1,4-glucan synthase activity in the seedlings was not completely restored by aeration under water, indicating that it is also affected by environmental factors other than low oxygen caused by submergence. It is conceivable that microgravity under water also affected the β-1,3 : 1,4-glucan synthase activity and the induced elongation growth of the coleoptiles (Hoson and Soga, 2003). This idea is supported by previous studies, which have shown that degradation of β-1,3 : 1,4-glucan in the rice coleoptile is accelerated under microgravity conditions, leading to stimulation of elongation growth (Hoson et al., 2002), and by the observation that, conversely, the degradation of glucan in the maize coleoptile is suppressed in a hypergravity environment, causing growth inhibition (Soga et al., 1999, 2000).
Another aspect of the submergence response studied here is that the deceleration of β-1,3 : 1,4-glucan synthesis may also contribute to the conservation of UDP-Glc under water, since β-1,3 : 1,4-glucan synthase consumes UDP-Glc as its substrate. Anaerobic stress induces expression of a sucrose synthase gene, SuSy, in rice seedlings (Ricard et al., 1991), which functions to generate fermentable sugars from sucrose under low oxygen conditions and to eventually supply ATP. Under the conditions investigated here, UGP-Glc saved by reduction of β-1,3 : 1,4-glucan synthesis may be converted to Glc 1-phosphate by the action of UDP-Glc pyrophosphorylase (EC 2·7·7·9), and then to Glc 6-phosphate by phosphoglucomutase (EC 5·4·2·2), which may then be metabolized to generate ATP through glycolysis. The present study thus suggests that in addition to the increased activity of SuSy, the deceleration of β-1,3 : 1,4-glucan synthesis is an important response of rice seedlings to efficiently generate ATP under low oxygen conditions. To address this possibility, the conservation of UDP-Glc and its conversion to fermentable hexoses such as Glc 6-phosphate under water should be examined.
ACKNOWLEDGEMENTS
This research was supported in part by a Grant for Ground Research for Space Utilization from the Japan Space Forum.
LITERATURE CITED
- Becker M, Vincent C, Reid JS. Biosynthesis of (1,3)(1,4)-β-glucan and (1,3)-β-glucan in barley (Hordeum vulgare L.). Properties of the membrane-bound glucan synthases. Planta. 1995;95:331–338. doi: 10.1007/BF00202589. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, et al. Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-D-glucans. Science. 2006;311:1940–1942. doi: 10.1126/science.1122975. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Kamisaka S, Hoson T. Breakdown of (1 → 3)(1 → 4)-β-D-glucans during development of rice coleoptiles in air and under water. Journal of Plant Physiology. 1999;a 155:234–239. [Google Scholar]
- Chen L, Kamisaka S, Hoson T. Suppression of (1 → 3)(1 → 4)-β-D-glucan turnover during light-induced inhibition of rice coleoptile growth. Journal of Plant Research. 1999;b 112:7–13. [Google Scholar]
- Fincher GB, Lock PA, Morgan MM, Lingelbach K, Wettenhall RE, Mercer JF, Brandt A, Thomsen KK. Primary structure of the (1 → 3),(1 → 4)-β-D-glucan 4-glucohydrolase from barley aleurone. Proceedings of the National Academy of Sciences of the USA. 1986;83:2081–2085. doi: 10.1073/pnas.83.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SP, Scott-Craig JS, Walton JD. Cellulose synthase-like genes of rice. Plant Physiology. 2002;128:336–340. doi: 10.1104/pp.010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoson T, Nevins DJ. β-D-Glucan antibodies inhibit auxin-induced cell elongation and changes in the cell wall of Zea coleoptile segments. Plant Physiology. 1989;90:1353–1358. doi: 10.1104/pp.90.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoson T, Soga K. New aspects of gravity responses in plant cells. International Review of Cytology. 2003;229:209–244. doi: 10.1016/s0074-7696(03)29005-7. [DOI] [PubMed] [Google Scholar]
- Hoson T, Soga K, Mori R, Saiki M, Nakamura Y, Wakabayashi K, Kamisaka S. Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant and Cell Physiology. 2002;43:1067–1071. doi: 10.1093/pcp/pcf126. [DOI] [PubMed] [Google Scholar]
- Hrmova M, Harvey AJ, Wang J, Shirley NJ, Jones GP, Stone BA, Høj PB, Fincher GB. Barley β-D-glucan exohydrolases with β-D-glucosidase activity. Purification, characterization, and determination of primary structure from a cDNA clone. Journal of Biological Chemistry. 1996;271:5277–5286. doi: 10.1074/jbc.271.9.5277. [DOI] [PubMed] [Google Scholar]
- Inouhe M, Nevins DJ. Auxin-enhanced glucan autohydrolysis in maize coleoptile cell walls. Plant Physiology. 1991;a 96:285–290. doi: 10.1104/pp.96.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouhe M, Nevins DJ. Inhibition of auxin-induced cell elongation of maize coleoptiles by antibodies specific for cell wall glucanases. Plant Physiology. 1991;b 96:426–431. doi: 10.1104/pp.96.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Kuroyama H, Takeuchi Y, Tsumuraya Y. Characterization of pectin methyltransferase from soybean hypocotyls. Planta. 2000;210:782–791. doi: 10.1007/s004250050680. [DOI] [PubMed] [Google Scholar]
- Kato Y, Nevins DJ. Enzymic dissociation of Zea shoot cell wall polysaccharides: II. Dissociation of (1 → 3),(1 → 4)-β-D-glucan by purified (1 → 3),(1 → 4)-β-D-glucan 4-glucanohydrolase from Bacillus subtilis. Plant Physiology. 1984;75:745–752. doi: 10.1104/pp.75.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T, Nakagawa N, Takeda K, Sakurai N. Auxin-induced elongation growth and expressions of cell wall-bound exo- and endo-β-glucanase in barley coleoptiles. Plant and Cell Physiology. 2000;41:1272–1278. doi: 10.1093/pcp/pcd056. [DOI] [PubMed] [Google Scholar]
- Loescher W, Nevins DJ. Auxin-induced changes in Avena coleoptile cell wall composition. Plant Physiology. 1972;50:556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfresh KC, Chourey PS. Anaerobiosis induces transcription but not translation of sucrose synthase in maize. Plant Physiology. 1988;87:542–546. doi: 10.1104/pp.87.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro N, Tsuji H, Suzuki Y, Tsutsumi N, Hirai A, Nakazono M. Analysis of expression of genes for mitochondrial aldehyde dehydrogenase in maize during submergence and following re-aeration. Breeding Science. 2006;56:365–370. [Google Scholar]
- Nakamura Y, Wakabayashi K, Hoson T. Temperature modulates the cell wall mechanical properties of rice coleoptiles by altering the molecular mass of hemicellulosic polysaccharides. Physiologia Plantarum. 2003;118:597–604. doi: 10.1034/j.1399-3054.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Ricard B, Rivoal J, Spiteri A, Pradet A. Anaerobic stress induces the transcription and translation of sucrose synthase in rice. Plant Physiology. 1991;95:669–674. doi: 10.1104/pp.95.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika H, Matsumura H, Takano T, Tsutsumi N, Nakazono M. A point mutation of Adh1 gene is involved in the repression of coleoptile elongation under submergence in rice. Breeding Science. 2006;56:69–74. [Google Scholar]
- Sakurai N, Masuda Y. Auxin-induced extension, cell wall loosening and changes in the wall polysaccharide content of barley coleoptile segments. Plant and Cell Physiology. 1978;19:1225–1233. [Google Scholar]
- Soga K, Harada K, Wakabayashi K, Hoson T, Kamisaka S. Increased molecular mass of hemicellulosic polysaccharides is involved in growth inhibition of maize coleoptiles and mesocotyls under hypergravity conditions. Journal of Plant Research. 1999;112:273–278. doi: 10.1007/pl00013881. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Hoson T, Kamisaka S. Hypergravity-induced increase in the apoplastic pH and its possible involvement in suppression of beta-glucan breakdown in maize seedlings. Australian Journal of Plant Physiology. 2000;27:967–972. [PubMed] [Google Scholar]
- Tsuchiya K, Urahara T, Konishi T, Kotake T, Tohno-oka T, Komae K, Kawada N, Tsumuraya Y. Biosynthesis of (1 → 3),(1 → 4)-β-glucan in developing endosperms of barley (Hordeum vulgare) Physiologia Plantarum. 2005;125:181–191. [Google Scholar]
- Urbanowicz BR, Rayon C, Carpita NC. Topology of the maize mixed linkage (1 → 3),(1 → 4)-β-d-glucan synthase at the Golgi membrane. Plant Physiology. 2004;134:758–768. doi: 10.1104/pp.103.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Komae K. A rice family 9 glycoside hydrolase isozyme with broad substrate specificity for hemicelluloses in type II cell walls. Plant and Cell Physiology. 2006;47:1541–1554. doi: 10.1093/pcp/pcl020. [DOI] [PubMed] [Google Scholar]
- Zarra I, Masuda Y. Growth and cell wall changes in rice coleoptiles growing under different conditions. I. Changes in turgor pressure and cell wall polysaccharides during intact growth. Plant and Cell Physiology. 1979;a 20:1117–1124. [Google Scholar]
- Zarra I, Masuda Y. Growth and cell wall changes in rice coleoptiles growing under different conditions. II. Auxin-induced growth in coleoptile segments. Plant and Cell Physiology. 1979;b 20:1125–1133. [Google Scholar]