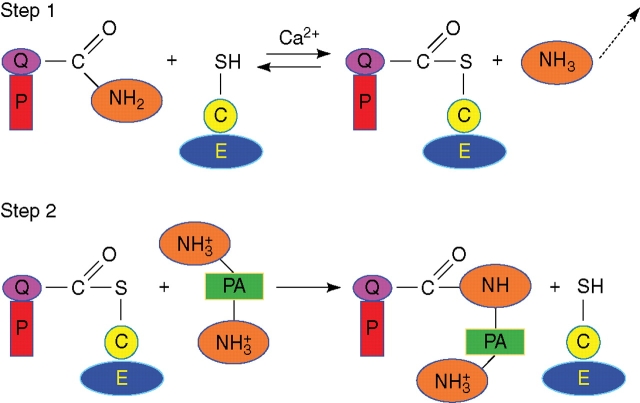
Fig. 1.
The two-step transamidase reaction of transglutaminase. All mammalian TGases belong to a superfamily of cysteine proteases, have structural homology and possess the catalytic triad of Cys-His-Asp/Asn; the reactivity of this Cys is activated by Ca2+, which causes a conformational change in the enzyme, allowing the access of the substrate to the binding site. Step 1: the active Ca2+-stabilized conformation of the enzyme forms a covalent intermediate between the active site thiol residue and a glutamyl residue in the protein substrate, releasing ammonia and activating the glutamine acyl moiety. Step 2: the active thioester undergoes an acyl transfer to a primary amine, in this case a polyamine, thus also introducing extra positive charges as PAs are protonated at physiological pH [or (not shown) to the lysyl residue of another protein]. A secondary cross-link might form between the free amine group of the bound polyamine and a glutamyl residue in another protein substrate thus forming bis-(γ-glutamyl)-PA derivatives (see Fig. 2). E, Transglutaminase; C, cysteine residue; PA, polyamine; P, protein substrate of transglutaminase; Q, glutamine residue.