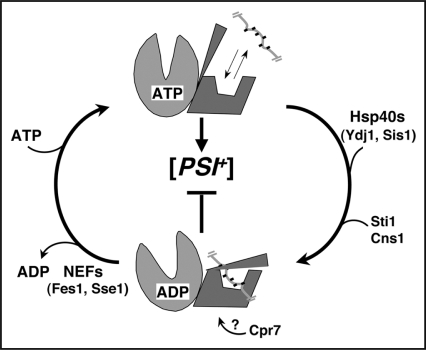
Figure 2.
Hsp70 reaction cycle and its regulation by co-chaperones. When ATP is bound to the nucleotide-binding domain (light gray) the substrate-binding domain (dark gray) is in an “open” conformation and rapidly exchanges substrate, which is indicated as a short hydrophobic segment of a larger protein. Hydrolysis of ATP to ADP (downward arching arrow) induces a conformational change in the substrate-binding and C-terminal domains that traps substrate. Exchange of nucleotides (upward arrow, ADP release allows rebinding of ATP) restores the open conformation and release of substrate. ATP hydrolysis is rate limiting and is stimulated by Hsp40s, Sti1 and Cns1. ADP release is enhanced by nucleotide exchange factors (NEFs). How Cpr7 influences Hsp70 is unknown but its effects on [PSI+], which are additive with those of Sti1, are consistent with Cpr7 stabilizing the ADP-bound state. Altering the cycle to favor the ADP-bound state (e.g., stimulating ATP hydrolysis or inhibiting nucleotide exchange) impairs [PSI+].