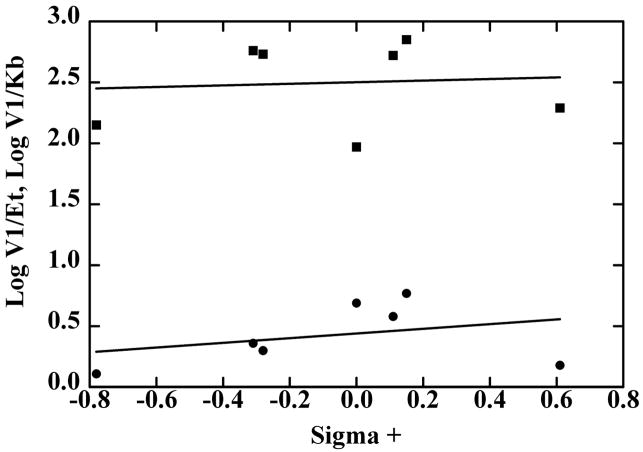
Fig. 1.
Hammett plots of the quantitative structure-activity relationship for oxidation of p-substituted benzyl alcohols by SceADH2. The data are from Table 6, where V1/Et (●) and V1/Kb (■) are plotted against the σ+ values for CH3O-, CH3- CH(CH3)2- H-, Cl-, Br-CF3-, from left to right. The lines were calculated by linear regression, but the slopes were not significantly determined: for log (V1/Et) slope is (0.19 ± 0.25)σ+, and for log (V1/Kb) slope is (0.06 ± 0.36)σ +.