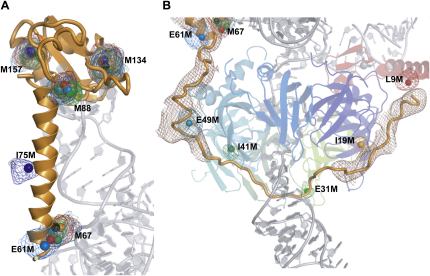
Figure 2.
Overlay of Selenium Peaks from Multiple Crystals Containing U1-70K SeMet Mutant Protein
(A) U1-70K residues 61–180 are shown as orange cartoon, with part of U1 snRNA, including SL1, shown in light gray. The selenium peak coordinates from anomalous maps of the eight U1-70K mutants are marked by colored spheres. The selenium anomalous maps are shown, all contoured at 3.5 σ and colored to match the spheres. Sphere diameter is ∼2 Å. As well as the four natural methionines (67, 88, 134, and 157), which have corresponding peaks in all the mutants, two of the mutant site peaks (E61M and I75M) are also shown. The colors are: wild-type, black; L9M, red; I19M, orange; E31M, light green; I41M, dark green; I49M, cyan; E61M, blue; I75M, dark blue.
(B) The path of the extended N terminus of U1-70K. Electron density attributed to U1-70K is shown in brown and contoured at 1 σ. Where density is absent, approximately between residues 24 and 45, a plausible path for the peptide is indicated based on the selenium positions of E31M and I41M. Selenium peaks and anomalous maps are as for (A). Near the selenium site of L9M, U1-70K is seen to interact with U1-C, which is red.