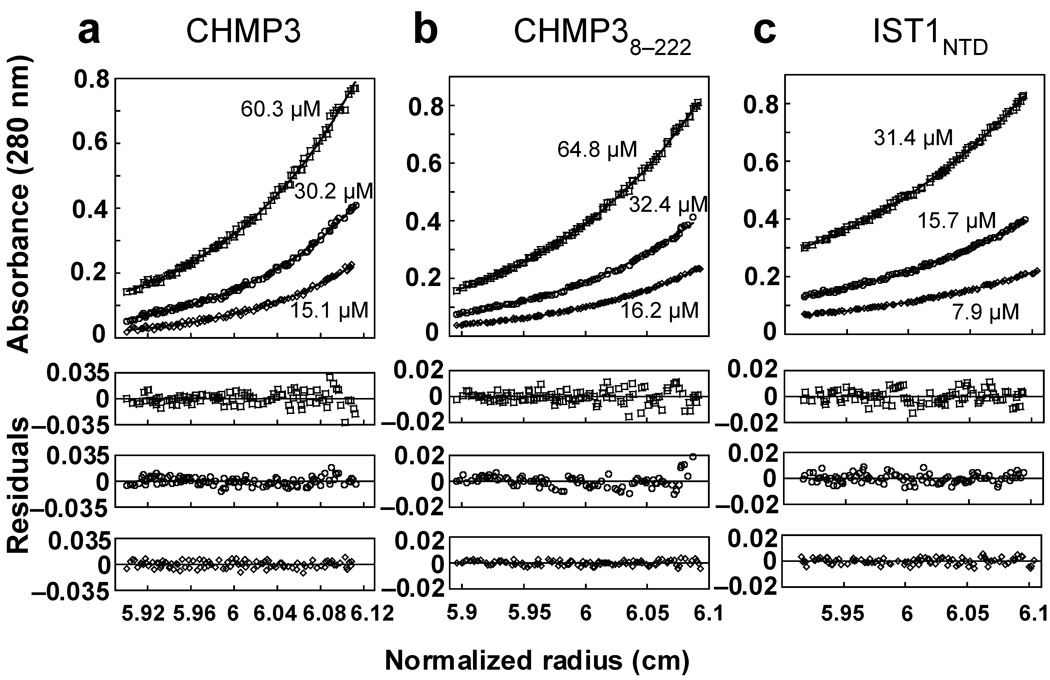
Figure 1.
CHMP3, CHMP38–222 and IST1NTD are monomers in solution. Equilibrium sedimentation distributions of recombinant CHMP3 (a), CHMP38–222 (b), and IST1NTD (c) (upper panels), and residual differences (lower panels), with data points shown in open symbols and the single species models shown as solid lines. Rotor speeds were 20,000 RPM and initial subunit protein concentrations are shown. Data sets were also collected at 24,000 RPM (not shown) and all of the data were globally fit to single species models in which the molecular weights were allowed to float during the refinement. Estimated molecular weights were: CHMP3, 25,840 Da (MWmonomer = 25,267 Da, Mobs/Mcalc = 1.02); CHMP38–222, 24,390 Da (MWmonomer = 24,663 Da, Mobs/Mcalc = 0.99); IST1NTD, 20,520 Da (MWmonomer = 21,791 Da, Mobs/Mcalc = 0.94).