Abstract
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily of transcription factors that respond to specific ligands by altering gene expression in a cell-, developmental- and sex-specific manner. Three subtypes of this receptor have been discovered (PPARα, β and γ), each apparently evolving to fulfill different biological niches. PPARs control a variety of target genes involved in lipid homeostasis, diabetes and cancer. Similar to other nuclear receptors, the PPARs are phosphoproteins and their transcriptional activity is affected by cross-talk with kinases and phosphatases. Phosphorylation by the mitogen-activated protein kinases (ERK- and p38-MAPK), Protein Kinase A and C (PKA, PKC), AMP Kinase (AMPK) and glycogen synthase kinase-3 (GSK3) affect their activity in a ligand-dependent or -independent manner. The effects of phosphorylation depend on the cellular context, receptor subtype and residue metabolized which can be manifested at several steps in the PPAR activation sequence including ligand affinity, DNA binding, coactivator recruitment and proteasomal degradation. The review will summarize the known PPAR kinases that directly act on these receptors, the sites affected and the result of this modification on receptor activity.
Introduction
The regulation of lipid metabolism and glucose utilization is critical for the maintenance of cellular energy homeostasis. Cells have developed several means to respond to internal and external stimuli that signal imbalances in metabolic processes and energy utilization. These include rapid responses such as phosphorylation events as well as relatively latent effects on gene transcription. Ultimately, the result of altered gene expression is the synthesis of new signaling molecules and enzymes that are able to meet the physiological needs of the cell and the organism. The Peroxisome Proliferator-Activated Receptors (PPARs) are members of the nuclear receptor (NR) superfamily that have evolved to be the biological sensors of altered lipid metabolism, in particular that of intracellular fatty acid levels. An interesting and somewhat surprising finding is that these lipid sensors are also profound regulators of cell growth, differentiation and apoptosis in a wide variety of cells. The multifaceted responses of PPARs are actually mediated by three subtypes expressed in different tissues and at different times in development. The PPAR subfamily (NR1C [1]) has been defined as PPARα (NR1C1), PPARβ (also called PPARδ and NUC1, NR1C2) and PPARγ (NR1C3), each with a possibility of different ligands, target genes and biological role. PPARs have been cloned in several species, including humans, rodents, amphibians, teleosts and cyclostoma [2]. The expression of PPARα, β and γ varies widely from tissue-to-tissue. In numerous cell types from either ectodermal, mesodermal, or endodermal origin, PPARs are coexpressed, although their concentration relative to each other varies widely [3]. PPARα is highly expressed in cells that have active fatty acid oxidation capacity including hepatocytes, cardiomyocytes, enterocytes, and the proximal tubule cells of kidney. PPARβ is expressed ubiquitously and often at higher levels than PPARα and γ. PPARγ, expressed predominantly in adipose tissue and the immune system, exists as two distinct protein forms γ1 and γ2, which arise by differential transcription start sites and alternative splicing [4].
Nuclear receptors can be activated in ligand-dependent and ligand-independent mechanisms. PPARs are activated by xenobiotics as well as endogenous fatty acids and their metabolites [5-8]. The term “activation” denotes an altering in the three dimensional structure of the receptor complex such that it is able to regulate gene expression. The physical alteration that is initiated by ligand binding may include events such as loss of heat shock proteins and chaperones, nuclear translocation, and protein turnover. Conformational changes of PPARα and γ have been observed using limited proteolysis [9, 10] and peptide interaction [11]. Binding of ligand to the PPARs also alters heat shock protein association [12, 13] and nuclear localization [14, 15] of the PPARs has also been noted.
Ligand-independent mechanisms of regulating NR activity including the PPARs is a relatively new area of study. Activation by ligand-independent mechanisms is most often associated with kinase-dependent processes and has been studied most extensively for the estrogen receptor-α (ERα) [16-18]. For example, the ERα contains two activation domains, AF-1 and AF-2, that are present in the A/B and E/F domain respectively; In the AF-1, Ser118 is phosphorylated by mitogen-activated protein kinase (MAPK or MEK), downstream of growth factor receptors and increases basal and ligand-induced activity of ERα [19]. Similarly, PPARα and PPARγ are phosphoproteins and MAPK (in particular ERK2), can modulate PPAR activity [20]; however, whether this is true ligand-independent modulation remains to be seen and may be particularly difficult to examine in light of the relatively high concentration of endogenous ligands present with the cell. None-the-less, it is clear that the activity of PPARα, β and γ is affected by phosphorylation status.
Phosphorylation of PPARs
PPARα
Growth factor signaling
The general approach used to examine cross-talk between kinase cascades and PPARs includes searches for consensus phosphorylation sites, manipulating growth factor signaling pathways with activators and inhibitors, site-directed mutagenesis of the receptor and finally examination of effects on biological activity. Although phosphopeptide mapping would give a more definitive look at the sites phosphorylated and it stoicheometry, this has proven to be a very difficult undertaking, due in part to low expression levels of PPARα. Several consensus phosphorylation sites for PPARα can be found (see Figure 1) including MAPK, casein kinase 2 (CK2), glycogen synthase kinase 3 (GSK3) and protein kinase A (PKA) and C (PKC) sites. It should be noted that these computer predictions are not very accurate and they always require detailed investigation. Growth factors such as TNFα, insulin and PDGF/EGF can affect PPARα activity, presumably via kinase cascades. Pretreatment of ML457 cells with PD98059, a MEK inhibitor, blocks peroxisome proliferator (PP)-induced c-fos, egr-1 and junB expression [21]. This data is supported by work reported previously [22] showing H7, a nonspecific kinase inhibitor, affected PP-induced gene expression. Thapsigargin and A23187 also affect PP-induced DNA synthesis, suggesting a role of calcium mobilization on PP-mediated gene expression. Activators of PKA can enhance mouse PPARα activity in the absence and the presence of exogenous ligands in transient transfection experiments [23]. PPARα can be inhibited by growth hormone (GH) via the Janus kinase-signal transducer and activator of transcription 5b (JAK2/STAT5b) signaling pathway [24]. Additionally, we have shown that inhibition of MAPK (with PD98059) signaling reduces PPARα activity whereas decreased PI3K activity (Ly2940004 or wortmannin) greatly enhances PPAR activity [25].
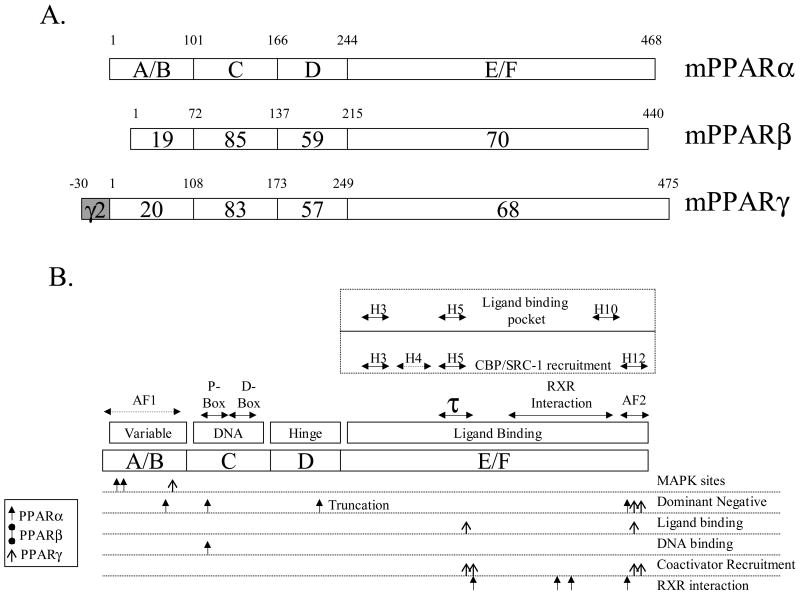
Figure 1.
Structure of PPARs. Panel A. Structure and functional domains of PPARα, β and γ. A/B is the hypervariable region containing the putative activation function-1 (AF-1) domain. PPARγ2 contains a 30 amino acid region that arises from differential promoter use and splicing. The C-domain is the most conserved and contains the DNA binding motif. The D-domain (hinge) is believed to allow for conformational change following ligand binding. The E/F domain contains the ligand-binding region of PPAR. Alignments and percent similarities were performed with MegAlign (DNAStar, Madison WI). Panel B. Detailed functional domains of PPARs. Above the outline for the hypothetical PPAR are the structural features of PPARs that have been deduced by sequence comparisons and crystallography. The AF-1 domain has not been fully characterized although it is known to reside in the A/B domain. The DNA binding motif contains two C4 zinc fingers, the proximal (P-box) and distal (D-box) boxes, which confer DNA binding and heterodimerization, respectively. Much has been learned about PPAR structure/function from recent crystallographic studies. PPAR E/F contains 13 alpha helices (H1-H12, H2′) and 4 short β strands [60] and helices 3, 5 and 10 forms the ligand-binding pocket. RXR interacts along several helices including H7-H10. The coactivator CBP interacts with H3-5 and H12 while SRC-1 associated with H3, H5 and H12. The τ1 domain contains a leucine-zipper-like heptad repeat [61]. The AF-2 domain is highly conserved among all PPARs and is intimately associated with ligand-induced transcriptional events. Much of the characterization of functional domains was performed using site-directed mutagenesis, as shown below the hypothetical PPAR. MAPK phosphorylation sites have been found at S12 and S21 in mouse PPARα and S122 in PPARγ2 [2]. Dominant negative (dn) PPARα results from mutations at L71, L123, and V444 [62] or in the naturally occurring truncated form of the receptor [63]. A dn PPARγ can be formed by mutating L468 and E471 of the human receptor [64]. Ligand binding mutants may arise from altering residues L319 or L469 of hPPARγ [65]. Similarly, DNA binding-devoid constructs are produced by mutating C122 [66] of PPARα. Specific interactions, such as those with SRC-1 and CBP, are targeted by mutating residues K301, V315, L468 or E471 of hPPARγ [65] while RXR association is lost by changing sites L433 [66], L370, L391 or D304 [61] of hPPARα. It is important to note that all domains work as a unified entity, with changes at the A/B terminus affecting ligand binding at the COOH, E/F domain [67] or in DNA binding [68]. Very little mutational analysis has been performed with PPARβ/γ, although the crystal structure reveals an E/F domain of this subtype to be very similar to PPARγ.
PPARα is phosphorylated exclusively on serine residues in vivo with no observable threonine or tyrosine activity1. Insulin increases the phosphorylation of PPARα, an effect that is associated with increased transcriptional activity [26]. Treatment of rat Fao cells with ciprofibrate increased the phosphorylation of PPARα [27]. In addition, treatment of these cells with phosphatase inhibitors decreased the activity of ciprofibrate-induced gene expression.
Extracellular receptor kinase-mitogen activated protein kinase (ERK-MAPK)
As mentioned above, insulin treatment of hepatocytes increased PPARα phosphorylation and activity. This increase in basal and induced activity is due to the insulin mediated ERK-MAPK activity [26]. The insulin-induced transactivation is due to the phosphorylation of two serines (12 and 21) in the A/B domain of human PPARα [28]. Co-transfection of MAPK phosphatase-1 (MKP-1) with PPARα resulted in a decrease in ligand inducible reporter activity [29], once again enforcing the role of the MAPK pathway on this NR. Interestingly, although the phosphorylation is in the A/B domain of PPARα, there is an effect on the ligand-dependent transactivation and not the ligand-independent AF-1 domain. This was demonstrated by the fact that the activity of heterologous PPARα A/B-Gal4 construct is not affected by the MEK inhibitor PD98059; also, introducing mutations into these constructs has no effect on ligand-independent activity (unpublished observations). The Wy14,643 induced activity of similar full length PPARα-Gal4 chimera was sensitive to PD98059 treatment. Thus, ERK-MEK phosphorylation affects intra-molecular communication whereby the phosphorylation status of the A/B domain affects the activity of the E/F region of the protein.
JNK and p38 MAPK
The p38 MAPK is activated by cytokines and is a member of the stress activated kinase family, affected by ischemia and hypoxia. In in vitro assays, p38 MAPK phosphorylates the A/B domain of PPARα (at serine 6, 12 or 21)[30]. This results in an enhancement of ligand-dependent transcriptional activity in cardiac myocytes as a consequence of increased interaction with the transcriptional coactivator PPARγ-coactivator-1α (PGC-1α) [30]. The p38 MAPK-enhanced recruitment of PGC-1 to PPARα is particularly germane in this model system whereby the coactivator has an important role in myocyte energy homeostasis [30]. In the rat cardiomyocyte cell line, the activation of ERK-MAPK decreased PPARα activity demonstrating the importance of cellular context [31]. Cerivastatin, an inhibitor of HMG CoA reductase, increases transcriptional activity of PPARα by inhibiting the formation of geranylgeranyl pyrophosphate [32]. The geranylgeranyl pyrophosphate pathway affects the prenylation of Rho family proteins (Rho, Rac and Cdc42) which in turn regulate the JNK- and the p38 -MAPK cascades. The geranylation of small G proteins is necessary for translocation of these proteins to the membrane and for their activation. By inhibiting Rho A small G protein activation, cerivastatin stimulates PPARα transcriptional activity by reducing its phosphorylation [32]. This provides an important means of cross-talk between two clinically relevant drug families (statins and fibrates) via phosphorylation.
Protein Kinase A
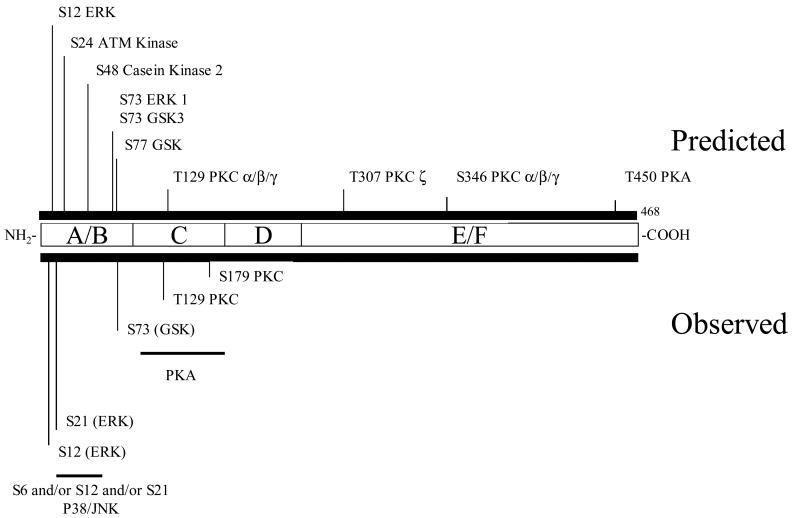
Activators of PKA such as cholera toxin (CT) enhance PPARα activity both in the absence and presence of exogenous ligands [23]. The main site of phosphorylation is located in the C-Domain, although the enhancement of activity requires the AF-2 domain [23], once again suggesting an effect of phosphorylation on intramolecular communication. In addition to the C-domain phosphorylation, an effect on PPARα-DNA interaction was observed with PKA activators. As shown in Figure 2, there are consensus PKA sites found in the C domain of PPARα, although to our knowledge, site directed mutagenesis has not been performed to confirm importance of this particular serine residue.
Figure 2. Structure of mouse PPARα and location of phosphorylation sites.
Serine or threonine consensus sites are marked with their location (i.e. S12 is serine at residue 12). The key shows the font associated with each kinase's consensus site. Abbreviations used: MAPK, mitogen activated protein kinase; PKC, protein kinase C; CK2, casein kinase 2; GSK3, glycogen synthase kinase 3; PKA, protein kinase A. Consensus sites scanned using Scansite (http://scansite.mit.edu/) under moderate stringency. The sequence was scanned for the following kinase sites: Akt_Kin, ATM_Kin, Cam_Kin2, Casn_Kin1, Casn_Kin2, Cdc2_Kin, Cdk5_Kin, Clk2_Kin, DNA_PK, Erk1_Kin, GSK3_Kin, p38_Kin, PKA_Kin, PKC_common, PKC_delta, PKC_epsilon, PKC_mu, PKC_zeta.
Protein Kinase C (PKC)
In vivo phosphorylation studies show that the level of phosphorylated PPARα is increased by treatment with the PP Wy-14,643 as well as the PKC activator phorbol myristol acetate (PMA) [33]. In addition, inhibitors of PKC decreased Wy-14,643-induced PPARα activity. Overexpressing PKCα, -β, -δ, and -ζ affected both basal and Wy-14,643-induced PPARα activity [33]. Four consensus PKC phosphorylation sites are contained within the DNA binding (C-domain) and hinge (D-domain) regions of rat PPARα (S110, T129, S142, and S179), and their contribution to receptor function has been examined [33, 34]. PKCα and β phosphorylate PPARα in human liver cells, at serines 179 and 230, increasing the ligand-induced PPARα transcriptional activity [34]. Mutation of T129 or S179 to alanine prevented heterodimerization of PPARα with RXRα, lowered the level of phosphorylation by PKCα and PKCδ in vitro, and lowered the level of phosphorylation of PPARα in transfected cells. In addition, the T129A mutation prevented PPARα from binding DNA in an electromobility shift assay [33].
5′-AMP-activated protein kinase (AMPK)
AMPK activation increases fatty acid oxidation in skeletal muscle by decreasing malonyl CoA concentrations. Activation of AMPK by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) increases mRNA expression of PPARα target genes and PGC-1 in cultured muscle cells and mouse skeletal muscle. Inhibition of PPARα and PGC-1 by siRNAs prevents AICAR-stimulated increase in fatty acid oxidation [35]. In a similar study, AMPK and p38 MAPK were involved in the activation of PPARα by adiponectin in muscle cells [36]; these effects were suppressed by the overexpression of a dominant-negative form of AMPK. Moreover, chemical inhibitors of AMPK and p38 MAPK potently repressed fatty acid oxidation and the induction of PPARα target gene expression by adiponectin. Taken together, these results suggest that adiponectin stimulates fatty acid oxidation in muscle cells by the sequential activation of AMPK, p38 MAPK, and PPARα.
Glycogen Synthase Kinase 3 (GSK3)
GSK3 is a constitutively active proline-directed serine/threonine-specific kinase that phosphorylates at SXXXS sites and is inhibited by phosphorylation [37]. GSK3 was identified as the kinase that phosphorylates and inactivates glycogen synthase, the final enzyme in glycogen synthesis; however, GSK3 has since been shown to play a role in multiple signaling pathways [38, 39]. In vitro kinase assays reveal that PPARα is a substrate of GSK3 being phosphorylated predominately at serine 73 in the A/B domain. The over expression of GSK3, as shown through pulse chase experiments, decreased the stability of PPARα. The decrease in stability of PPARα was abrogated by mutating serine 73 in PPARα. The change in stability of PPARα is mediated via degradation of the ubiquitin proteasome system and suggests that the ubiquitin proteasome pathway is important for the rapid degradation of PPARα facilitated by GSK3 (unpublished results). With GSK3 and PPARα both being important metabolic players, this link may be important to the study of diseases such as diabetes or obesity.
PPARβ/δ
As with many areas of research, PPARβ/δ is the least studied subtype in terms of post-translational modification and phosphorylation. Similar to PPARα, PPARβ/δ has several consensus phosphorylation (Figure 3). Both cAMP and PKA activators (i.e. CT) increase ligand-activated and basal activity of PPARβ/δ [40-42]. It has been proposed that this affect of PKA is due to effects on recruitment of Nuclear receptor CORepressor (NcoR) and silencing mediator for retinoid and thyroid hormone receptor (SMRT) corepressors [40-42]. The specific PPARβ/δ agonist (GW501516) increases the prostaglandin E2 receptor subtype EP4 mRNA and protein levels [43]. Wortmannin, a selective inhibitor of phosphatidylinositol 3-kinase (PI3-K), but not an inhibitor of Erk, eliminated the effect of GW501516 on EP4 expression. Preincubation of myotubes with the p38 MAPK inhibitor SB203580 reduced insulin- and PPARβ/δ-mediated increase in glucose uptake, whereas PD98059 had no effect [44]. Given the emerging appreciation for the importance of PPARβ/δ in diseases such as obesity, diabetes and inflammation, more studies on phosphorylation and growth factor signaling is well warranted.
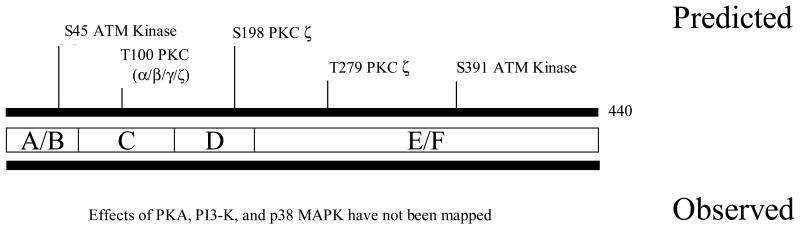
Figure 3. Structure of mouse PPARβ/δ and location of phosphorylation sites.
See Figure 2 for details.
PPARγ
Growth factor signaling
PPARγ is also a phosphoprotein phosphorylated by activators of MAPK, like insulin; however, this modification decreases transcriptional activity of PPARγ [45, 46]. Growth factor platelet derived growth factor (PDGF) treatment of adipocytes in culture decreases the transcriptional activity of PPARγ1 [47]. This receptor undergoes EGF-stimulated MEK-dependent phosphorylation and co-transfection of adipocytes with a constitutively active MEK decreases PPARγ transcriptional activity. In vitro assays demonstrate that ERK2 and JNK are able to phosphorylate PPARγ2 [46], which may help explain the effects of EGF and TNFα, respectively, on gene expression. Insulin and a PPARγ ligand (troglitazone, TZD) act synergistically to increase the expression of an adipocyte specific gene, aP2 [48].
Extracellular receptor kinase-mitogen activated protein kinase (ERK-MAPK)
Transfection with a dominant negative MEK results in a decrease in both insulin and TZD's effects on PPARγ activity, indicating MAPK is involved in the cross talk between PPARγ and insulin [48]. In vitro assays demonstrate that ERK2 and JNK are able to phosphorylate PPARγ2 [46]. The MAPK phosphorylation site, which can be used by both ERK- and JNK-MAPK [49], was mapped at serine 82 of mouse PPARγ1, which corresponds to serine 112 of mouse PPARγ2 [50]. Substitution of this serine by alanine (S82A) leads to a loss of PDGF mediated repression of PPARγ activity [47]. Human PPARγ1 phosphorylation at this site (S84) inhibits both its ligand-dependent and -independent transactivating function. The S84A mutant showed an increase in the AF-1 transcriptional activity of PPARγ [46]. Treatment of macrophages with TGFβ1 increases PPARγ phosphorylation and decreases TZD-induced CD36 expression via an activation of the ERK-MAPK pathway [51]. Mutation of the main MAPK site of phosphorylation in PPARγ2 (S112D) exhibits a decreased ligand-binding affinity [50]. Similar to that noted for PPARα, this suggests interdomain communications between the AF-1 domain and ligand-binding pocket. Limited protease digestion shows the unliganded PPARγ2 and the S112D mutant have different sensitivity; thus, the phosphorylation status of serine 112 plays a role in the conformation of the unliganded receptor which regulates the affinity of PPARγ for its ligands and affects coactivator recruitment ability [50]. It has been proposed that phosphorylation-mediated inhibition of transcriptional activity of nuclear receptors is an important “off-switch” of ligand-induced activity (reviewed in [52]). Extracellular signals which activate intracellular phosphorylation pathways can influence the degradation process of PPARγ [53]. For example, treatment of cells with an inhibitor of MEK kinases inhibits the degradation of PPARγ. However, not all phosphorylation events are inhibitory and enhance proteosomal degradation and care must be taken when making a global speculation.
Substitution of proline to glutamine at position 115 of the human receptor renders PPARγ constitutively active through the modulation of the MAPK-dependent phosphorylation status of serine 114 [54]. Subjects carrying this mutation are extremely obese, but surprisingly show a lesser insulin resistance than expected. In mice homozygous for the S112A mutant (homologous to human S114) [55] there is protection against diet induced obesity. This may be due to changes in adipocyte function such as secretion of adiponectin and leptin. Overall, prevention of PPARγ phosphorylation leads to an improvement of insulin sensitivity mainly due to increased glucose disposal in muscle, which is similar to TZD treatment [55].
Protein Kinase A
As was seen with PPARα and PPARβ, activation of PKA with CT increased the basal and ligand-induced activity of PPARγ [40]. The details of this activation were not pursued to the same extent as PPARα. Treatment with PKA stimulators markedly increased while MEK and PI 3-kinase overexpression resulted in a decrease in PPARγ activity [56]. Clearly, more studies are required to understand the PKA-induced phosphorylation of PPARγ.
Protein Kinase C (PKC)
One of the major adverse effects of PPARγ agonists is fluid retention and edema, resulting from an unknown mechanisms. A recent study has shown that TZDs effects on edema and weight are partially due to an adipose tissue-selective activation of PKC and vascular permeability that may be prevented by PKCβ inhibition [57]. Although not directly studied, it is possible that PKC can directly phosphorylate PPARγ (there are several consensus site, Figure 4), thus leading to altered activity.
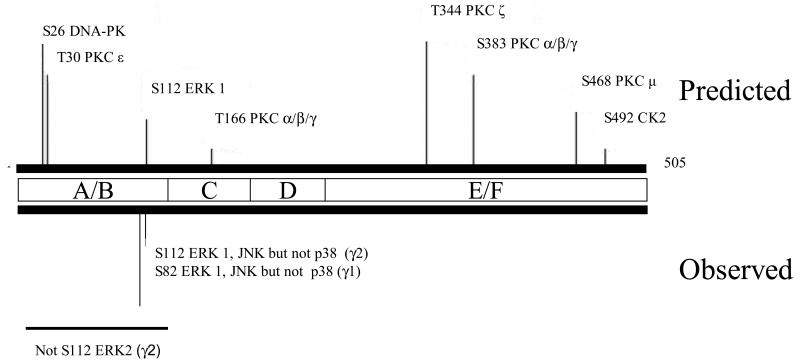
Figure 4. Structure of mouse PPARγ2 and location of phosphorylation sites.
See Figure 2 for details.
5′-AMP-activated protein kinase (AMPK)
Similar to PPARα, PPARγ is sensitive to AMPK activity. PPARγ phosphorylation by AMPK represses both the ligand-dependent and -independent transactivating function of the receptor [58]. It has been proposed that one of the mechanisms by which TZDs improve insulin sensitivity is by increasing the expression and release of adiponectin, an adipokine that activates AMPK. However, it has been reported that TZDs also acutely activate AMPK in skeletal muscle and other tissues [59]. Thus, some of TZDs effects may be PPARγ-independent.
Summary
PPARs are members of the steroid hormone receptor superfamily that respond to changes in lipid and glucose homeostasis. Thus far, three subtypes (α, β and γ) have been identified in many species including humans. The manner in which PPARs regulate gene expression is an area of intense research and appears to be similar for α, β and γ, regardless of the species examined. For example, upon activation with ligand, PPARs associate with the retinoid-X-receptor (RXR) and bind to specific response elements found on target genes. The subsequent alteration in gene expression by the PPARs is influenced by kinases, cofactors and other tissue specific factors. Detailed examination of the structure-function of the PPARs allows for an understanding of certain polymorphisms within the human population and may also aid in the design of new therapeutic agents.
The biological niches of PPARα, β and γ are distinct, yet they have many overlapping functions. PPARα is the cognate receptor for peroxisome proliferators as well as certain fatty acid and their metabolites. Through the extensive use of the PPARα null mouse model, it is evident that this receptor plays a key role in lipid homeostasis, particularly in the fasted state. Important fatty acid oxidation enzymes, in peroxisomes and elsewhere, are regulated by PPARα. PPARγ has received much attention as the target for anti-diabetic drugs, but also plays a role in responding to endogenous compounds such as prostaglandin J2. The ability of ectopically-expressed PPARγ to induce differentiation of adipocytes, macrophages and other cells, underscores the importance of this protein in regulating cell fate and implies a role beyond fatty acid metabolism. The embryonic lethality of the PPARγ-null mouse has made identifying definitive biological roles of this particular subtype difficult. PPARβ remains a somewhat underappreciated member of this subfamily of receptors. The endogenous ligands for PPARβ are, as a group, relatively weak activators, but they include various fatty acids. The phenotype of the PPARβ-null suggests an important role in lipid homeostasis and this protein has received attention as a downstream target of growth regulatory genes, in particular in the colon.
It is interesting to note that many biological functions of the PPARs are redundant such as their ability to affect fatty acid metabolism, although certain phosphorylation events have distinct effects. For example, the MAPK pathway activates PPARα in hepatocytes, whereas it inhibits PPARγ activity in adipocytes. Similarly, PPARγ phosphorylation by AMPK represses both the ligand-dependent and -independent transactivating function of the receptor; the opposite is seen with AMPK activation of PPARα in heart. In contrast, PKA phosphorylation positively affects the activity of all three PPAR subtypes. The effects of phosphorylation can be seen in many aspects of PPARs' mechanism of action including ligand, DNA and coactivator binding. An interesting phenomena that has been observed is that kinase activity can affect the intramolecular communication between the A/B and E/F domains; hence, metabolism of the AF-1 affects AF-2 activity. The action of kinase cascades on PPAR activity reflects the complexity of signaling cascades in a biological system. A goal of on-going research in this area is to understand the cross-talk between the PPAR and kinase cascades to better design treatments for diseases such as diabetes, inflammation and cancer.
Acknowledgments
Funded by NIH ES007799 (J.V.H.) and a Bristol Myers Squibb fellowship (K.A.B.)
Footnotes
G.H. Perdew, Penn State University, unpublished results.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.N.R.N. Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 2.Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448:121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- 3.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, - beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 4.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 5.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 6.Gottlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 8.Sher T, Yi HF, McBride OW, Gonzalez FJ. cDna cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993;32:5598–5604. doi: 10.1021/bi00072a015. [DOI] [PubMed] [Google Scholar]
- 9.Berger J, Bailey P, Biswas C, Cullinan CA, Doebber TW, Hayes NS, Saperstein R, Smith RG, Leibowitz MD. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-gamma: binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology. 1996;137:4189–4195. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- 10.Dowell P, Peterson VJ, Zabriskie TM, Leid M. Ligand-induced peroxisome proliferator-activated receptor alpha conformational change. J Biol Chem. 1997;272:2013–2020. doi: 10.1074/jbc.272.3.2013. [DOI] [PubMed] [Google Scholar]
- 11.Tien ES, Hannon DB, Thompson JT, Vanden Heuvel JP. Examination of ligand-dependent coactivator recruitment by Peroxisome Proliferator-Activated Receptor-α. PPAR Research. 2006;1 doi: 10.1155/PPAR/2006/69612. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumanasekera WK, Tien ES, Turpey R, Vanden Heuvel JP, Perdew GH. Evidence That Peroxisome Proliferator-activated Receptor alpha Is Complexed with the 90-kDa Heat Shock Protein and the Hepatitis Virus B X-associated Protein 2. J Biol Chem. 2003;278:4467–4473. doi: 10.1074/jbc.M211261200. [DOI] [PubMed] [Google Scholar]
- 13.Sumanasekera WK, Tien ES, Davis JW, 2nd, Turpey R, Perdew GH, Vanden Heuvel JP. Heat shock protein-90 (Hsp90) acts as a repressor of peroxisome proliferator-activated receptor-alpha (PPARalpha) and PPARbeta activity. Biochemistry. 2003;42:10726–10735. doi: 10.1021/bi0347353. [DOI] [PubMed] [Google Scholar]
- 14.Patel H, Truant R, Rachubinski RA, Capone JP. Activity and subcellular compartmentalization of peroxisome proliferator-activated receptor alpha are altered by the centrosome-associated protein CAP350. J Cell Sci. 2005;118:175–186. doi: 10.1242/jcs.01600. [DOI] [PubMed] [Google Scholar]
- 15.Akiyama TE, Baumann CT, Sakai S, Hager GL, Gonzalez FJ. Selective intranuclear redistribution of PPAR isoforms by RXR alpha. Mol Endocrinol. 2002;16:707–721. doi: 10.1210/mend.16.4.0797. [DOI] [PubMed] [Google Scholar]
- 16.El-Tanani MK, Green CD. Two separate mechanisms for ligand-independent activation of the estrogen receptor. Mol Endocrinol. 1997;11:928–937. doi: 10.1210/mend.11.7.9939. [DOI] [PubMed] [Google Scholar]
- 17.Smith CL. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod. 1998;58:627–632. doi: 10.1095/biolreprod58.3.627. [DOI] [PubMed] [Google Scholar]
- 18.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. Embo J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 19.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzer D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen- activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 20.Vanden Heuvel JP. Peroxisome proliferator-activated receptors (PPARS) and carcinogenesis. Toxicol Sci. 1999;47:1–8. doi: 10.1093/toxsci/47.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Rokos CL, Ledwith BJ. Peroxisome proliferators activate extracellular signal-regulated kinases in immortalized mouse liver cells. J Biol Chem. 1997;272:13452–13457. doi: 10.1074/jbc.272.20.13452. [DOI] [PubMed] [Google Scholar]
- 22.Ledwith BJ, Johnson TE, Wagner LK, Pauley CJ, Manam S, Galloway SM, Nichols WW. Growth regulation by peroxisome proliferators: opposing activities in early and late G1. Cancer Res. 1996;56:3257–3264. [PubMed] [Google Scholar]
- 23.Lazennec G, Canaple L, Saugy D, Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou YC, Waxman DJ. Cross-talk between janus kinase-signal transducer and activator of transcription (JAK-STAT) and peroxisome proliferator-activated receptor-alpha (PPARalpha) signaling pathways. Growth hormone inhibition of pparalpha transcriptional activity mediated by stat5b. J Biol Chem. 1999;274:2672–2681. doi: 10.1074/jbc.274.5.2672. [DOI] [PubMed] [Google Scholar]
- 25.Vanden Heuvel JP. Peroxisome proliferator-activated receptors: a critical link among fatty acids, gene expression and carcinogenesis. J Nutr. 1999;129:575S–580S. doi: 10.1093/jn/129.2.575S. [DOI] [PubMed] [Google Scholar]
- 26.Shalev A, Siegrist-Kaiser CA, Yen PM, Wahli W, Burger AG, Chin WW, Meier CA. The peroxisome proliferator-activated receptor alpha is a phosphoprotein: regulation by insulin. Endocrinology. 1996;137:4499–4502. doi: 10.1210/endo.137.10.8828512. [DOI] [PubMed] [Google Scholar]
- 27.Passilly P, Schohn H, Jannin B, Malki MC, Boscoboinik D, Dauca M, Latruffe N. Phosphorylation of peroxisome proliferator-activated receptor alpha in rat Fao cells and stimulation by ciprofibrate. Biochem Pharmacol. 1999;58:1001–1008. doi: 10.1016/s0006-2952(99)00182-3. [DOI] [PubMed] [Google Scholar]
- 28.Juge-Aubry CE, Hammar E, Siegrist-Kaiser C, Pernin A, Takeshita A, Chin WW, Burger AG, Meier CA. Regulation of the transcriptional activity of the peroxisome proliferator-activated receptor alpha by phosphorylation of a ligand-independent trans-activating domain. J Biol Chem. 1999;274:10505–10510. doi: 10.1074/jbc.274.15.10505. [DOI] [PubMed] [Google Scholar]
- 29.Vanden Heuvel JP, Kreder D, Belda B, Hannon DB, Nugent CA, Burns KA, Taylor MJ. Comprehensive analysis of gene expression in rat and human hepatoma cells exposed to the peroxisome proliferator WY14,643. Toxicol Appl Pharmacol. 2003;188:185–198. doi: 10.1016/s0041-008x(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 30.Barger PM, Browning AC, Garner AN, Kelly DP. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 31.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin G, Duez H, Blanquart C, Berezowski V, Poulain P, Fruchart JC, Najib-Fruchart J, Glineur C, Staels B. Statin-induced inhibition of the Rho-signaling pathway activates PPARalpha and induces HDL apoA-I. J Clin Invest. 2001;107:1423–1432. doi: 10.1172/JCI10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray JP, Burns KA, Leas TL, Perdew GH, Vanden Heuvel JP. Regulation of peroxisome proliferator-activated receptor alpha by protein kinase C. Biochemistry. 2005;44:10313–10321. doi: 10.1021/bi050721g. [DOI] [PubMed] [Google Scholar]
- 34.Blanquart C, Mansouri R, Paumelle R, Fruchart JC, Staels B, Glineur C. The protein kinase C signaling pathway regulates a molecular switch between transactivation and transrepression activity of the peroxisome proliferator-activated receptor alpha. Mol Endocrinol. 2004;18:1906–1918. doi: 10.1210/me.2003-0327. [DOI] [PubMed] [Google Scholar]
- 35.Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 37.ter Haar E, Coll JT, Austen DA, Hsiao HM, Swenson L, Jain J. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat Struct Biol. 2001;8:593–596. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- 38.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 40.Lazennec G, Canaple L, Saugy D, Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen JB, Zhang H, Rasmussen TH, Petersen RK, Flindt EN, Kristiansen K. Peroxisome proliferator-activated receptor delta (PPARdelta)-mediated regulation of preadipocyte proliferation and gene expression is dependent on cAMP signaling. J Biol Chem. 2001;276:3175–3182. doi: 10.1074/jbc.M005567200. [DOI] [PubMed] [Google Scholar]
- 42.Krogsdam AM, Nielsen CA, Neve S, Holst D, Helledie T, Thomsen B, Bendixen C, Mandrup S, Kristiansen K. Nuclear receptor corepressor-dependent repression of peroxisome-proliferator-activated receptor delta-mediated transactivation. Biochem J. 2002;363:157–165. doi: 10.1042/0264-6021:3630157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han S, Ritzenthaler JD, Wingerd B, Roman J. Activation of peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) increases the expression of prostaglandin E2 receptor subtype EP4. The roles of phosphatidylinositol 3-kinase and CCAAT/enhancer-binding protein beta. J Biol Chem. 2005;280:33240–33249. doi: 10.1074/jbc.M507617200. [DOI] [PubMed] [Google Scholar]
- 44.Kramer DK, Al-Khalili L, Perrini S, Skogsberg J, Wretenberg P, Kannisto K, Wallberg-Henriksson H, Ehrenborg E, Zierath JR, Krook A. Direct activation of glucose transport in primary human myotubes after activation of peroxisome proliferator-activated receptor delta. Diabetes. 2005;54:1157–1163. doi: 10.2337/diabetes.54.4.1157. [DOI] [PubMed] [Google Scholar]
- 45.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 46.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen- activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 47.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Berger J, Zhou G, Elbrecht A, Biswas S, White-Carrington S, Szalkowski D, Moller DE. Insulin- and mitogen-activated protein kinase-mediated phosphorylation and activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 1996;271:31771–31774. doi: 10.1074/jbc.271.50.31771. [DOI] [PubMed] [Google Scholar]
- 49.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140:392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 50.Shao DL, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 51.Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM, Jr, Nicholson AC. Transforming growth factor-beta1 (TGF-beta1) and TGF-beta2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-gamma. J Biol Chem. 2000;275:1241–1246. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- 52.Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 53.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 54.Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 55.Rangwala SM, Rhoades B, Shapiro JS, Rich AS, Kim JK, Shulman GI, Kaestner KH, Lazar MA. Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 56.Akahoshi T, Namai R, Murakami Y, Watanabe M, Matsui T, Nishimura A, Kitasato H, Kameya T, Kondo H. Rapid induction of peroxisome proliferator-activated receptor gamma expression in human monocytes by monosodium urate monohydrate crystals. Arthritis Rheum. 2003;48:231–239. doi: 10.1002/art.10709. [DOI] [PubMed] [Google Scholar]
- 57.Sotiropoulos KB, Clermont A, Yasuda Y, Rask-Madsen C, Mastumoto M, Takahashi J, Della Vecchia K, Kondo T, Aiello LP, King GL. Adipose-specific effect of rosiglitazone on vascular permeability and protein kinase C activation: novel mechanism for PPARgamma agonist's effects on edema and weight gain. Faseb J. 2006;20:1203–1205. doi: 10.1096/fj.05-4617fje. [DOI] [PubMed] [Google Scholar]
- 58.Leff T. AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins. Biochem Soc Trans. 2003;31:224–227. doi: 10.1042/bst0310224. [DOI] [PubMed] [Google Scholar]
- 59.LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 60.Gampe RTJ, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE. Asymetry in the PPARγ/RXRα cyrstal structure reveals the molecular basis of heterodimerization among nuclear receptors. Molecular Cell. 2000;5:545–555. doi: 10.1016/s1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- 61.Gorla-Bajszczak A, Juge-Aubry C, Pernin A, Burger AG, Meier CA. Conserved amino acids in the ligand-binding and tau(i) domains of the peroxisome proliferator-activated receptor alpha are necessary for heterodimerization with RXR. Mol Cell Endocrinol. 1999;147:37–47. doi: 10.1016/s0303-7207(98)00217-2. [DOI] [PubMed] [Google Scholar]
- 62.Roberts RA, James NH, Woodyatt NJ, Macdonald N, Tugwood JD. Evidence for the suppression of apoptosis by the peroxisome proliferator activated receptor alpha (PPAR alpha) Carcinogenesis. 1998;19:43–48. doi: 10.1093/carcin/19.1.43. [DOI] [PubMed] [Google Scholar]
- 63.Gervois P, Torra IP, Chinetti G, Grotzinger T, Dubois G, Fruchart JC, Fruchart-Najib J, Leitersdorf E, Staels B. A truncated human peroxisome proliferator-activated receptor alpha splice variant with dominant negative activity. Mol Endocrinol. 1999;13:1535–1549. doi: 10.1210/mend.13.9.0341. [DOI] [PubMed] [Google Scholar]
- 64.Gurnell M, Wentworth JM, Agostini M, Adams M, Collingwood TN, Provenzano C, Browne PO, Rajanayagam O, Burris TP, Schwabe JW, Lazar MA, Chatterjee VK. A dominant-negative peroxisome proliferator-activated receptor gamma (PPARgamma) mutant is a constitutive repressor and inhibits PPARgamma- mediated adipogenesis. J Biol Chem. 2000;275:5754–5759. doi: 10.1074/jbc.275.8.5754. [DOI] [PubMed] [Google Scholar]
- 65.Chen S, Johnson BA, Li Y, Aster S, McKeever B, Mosley R, Moller DE, Zhou G. Both coactivator LXXLL motif-dependent and -independent interactions are required for peroxisome proliferator-activated receptor gamma (PPARgamma) function. J Biol Chem. 2000;275:3733–3736. doi: 10.1074/jbc.275.6.3733. [DOI] [PubMed] [Google Scholar]
- 66.Juge-Aubry C, Pernin A, Favez T, Burger AG, Wahli W, Meier CA, Desvergne B. DNA binding properties of peroxisome proliferator-activated receptor subtypes on various natural peroxisome proliferator response elements. Importance of the 5'-flanking region. J Biol Chem. 1997;272:25252–25259. doi: 10.1074/jbc.272.40.25252. [DOI] [PubMed] [Google Scholar]
- 67.Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 68.Deeb SS, Fajas L, Nemoto M, Pihlajamaeki J, Mykkaenen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nature Genet. 1998;20:284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]