Abstract
The Extracellularly Regulated Kinase/Mitogen Activated Protein Kinase (ERK/MAPK) signaling pathway is a critical regulator of cellular processes in adult and developing tissues. Depending on the cellular context, MAPK cascade can act as a rheostat, a switch, or an oscillator. The highly conserved structure of the cascade does not imply a rigid function, as was suggested by the early mathematical models of MAPK signaling, and can instead produce a wide range of input-output maps. Given a large number of pathway components and modes of regulation, it is essential to establish experimental systems that will allow both manipulating the MAPK cascade and monitoring its dynamics. The terminal patterning system in the Drosophila embryo appears to be ideally suited for this purpose. Our recent experiments characterized dynamics of the MAPK phosphorylation gradient in the terminal system and proposed that it is regulated by a cascade of diffusion-trapping modules. Here we discuss a biophysical model that can describe the observed dynamics and guide future experiments for exploring the relative importance of multiple layers of MAPK cascade regulation.
Keywords: morphogen gradient, enzymatic cascade, signal transduction
Introduction
The Extracellularly Regulated Kinase/Mitogen Activated Protein Kinase (ERK/MAPK) signaling pathway is a critical regulator of cellular processes in developing and adult tissues.1–4 At the core of the pathway is a three-stage cascade of phosphorylation-dephosphorylation reactions that culminates in a double phosphorylation of MAPK, the key serine/threonine kinase at the last stage of the cascade (Fig. 1A). By acting on cytoplasmic and nuclear substrates, phosphorylated MAPK controls cellular processes, such as cell division and differentiation. The MAPK pathway is one of the most extensively modeled signaling systems.5–7 The goals of mathematical models of MAPK and other signaling pathways are to integrate the results of genetic, biochemical and cellular studies, and to predict the dynamics of signaling and its effects on cellular processes.
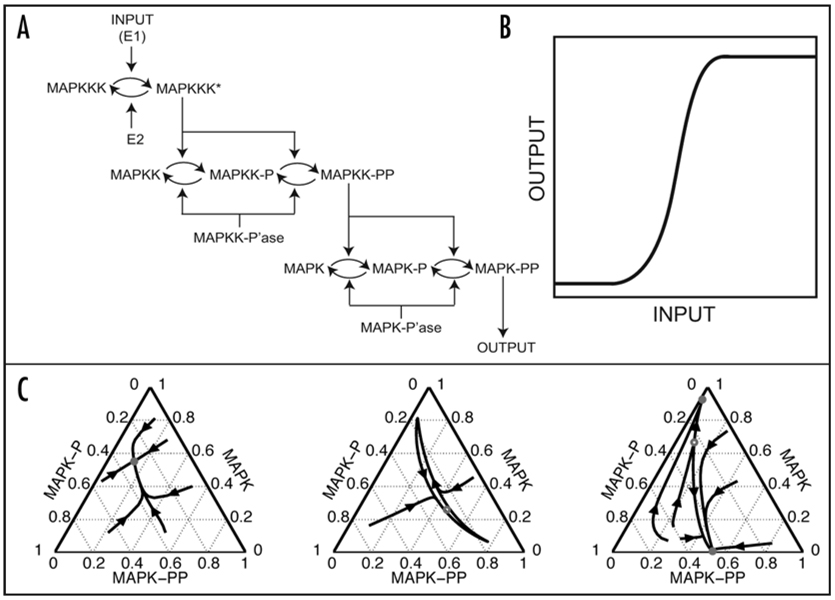
Figure 1.
(A) The three-stage structure of the MAPK cascade.8 (B) A schematic of the ultrasensitive input-output (I/O) map: the output is switched between the “off” and “on” states within a very narrow ranges of inputs. Such an input-output map can be described using the Hill function: O = [In/(Kn + In)], where n > 1; I and O denote the input and output, respectively, and K is the value of the input that leads to the half-maximal value of the output. The 1996 analysis by Huang and Ferrell suggested that n ~ 4.8 for the I/O map of MAPK cascade in the model. Importantly, there is a single value of output for every input. (C) Statistical analysis of the I/O maps in the Huang-Ferrell model revealed that, in addition to being single-valued, they can also be bistable: there are two stable values of the output that can be realized for the same value of input. Furthermore, the cascade can also act as an oscillator, which means that the output of the cascade (the extent of MAPK phosphorylation) will undergo undamped oscillations. The three types of I/O maps, single-valued (left), bistable (right), and oscillatory (middle), show the fractions of MAPK in three possible phosphorylation stages (unphosphorylated, once- and twice-phosphorylated). Reproduced from Qiao et al. PLoS Comp Biol 2007; 3:1819–26.
The first model of the MAPK cascade was developed by Huang and Ferrell, who used their model as a basis for linking the structure of the cascade and its dynamics (Fig. 1A). Their model represents an idealized situation where the enzymes are placed in a well-mixed system with nonlimiting amounts of ATP and other co-factors. Based on the mass-action kinetics, the model describes the dynamics of 22 species participating in ten reactions.8 Huang and Ferrell hypothesized that the three-tiered structure of the cascade controls its steady-state input-output behavior. Since double phosphorylation of MAPK is necessary for its catalytic activity, the fraction of MAPK in its double phosphorylated form was considered the output of the cascade. To compute the input-output maps, the top-layer of the cascade was activated to some constant level and the downstream components were allowed to reach a steady state level of activation. Clearly, to perform such an analysis, one has to specify model parameters, such as the starting amounts of kinases and phosphatases and the rate constants of enzymatic reactions.
All in all, the model had 36 parameters, each of which was given within a 25-fold range, and calculation of input-output maps was based on sampling of their values from these ranges. Briefly, one generates a 36-dimensional vector of model parameters and then uses it to find the values of the output of the cascade for multiple values of inputs. Every one of such calculations generates a single input-output map. Based on calculations with hundreds of randomly generated parameter sets, Huang and Ferrell found that all of the input-output maps in their model are ultrasensitive: there is a single output for every input, but the output can switch from “off” to “on” within a very narrow range of inputs (Fig. 1B). Recently, however, we have demonstrated that, within a very wide range of model parameters, the same model can behave as an irreversible switch and as an oscillator (Fig. 1C).9
Given the fact that even the simplest models of this important pathway can lead to complex dynamics, it is essential to experimentally determine what types of dynamics are actually realized in vivo. We are using the terminal patterning system in the early Drosophila embryo as an experimental model for monitoring the dynamics of MAPK signaling under a wide range of genetic and environmental conditions. Our long-term goal is to explore the regulation of MAPK signaling by multiple cellular and biochemical processes and to develop increasingly mechanistic models of this pathway. Here we review the first results of our approach that is based on a combination of previously developed genetic tools, quantitative imaging and biophysical models.
Quantitative Analysis of MAPK Activation in the Early Embryo
The development of the unsegmented regions of the Drosophila embryo depends on the localized activation of the Torso receptor tyrosine kinase. Torso is uniformly distributed throughout the plasma membrane of the early embryo but activated only at the poles, by its locally processed ligand (Trunk), where it signals through the MAPK cascade.10 Importantly, terminal patterning operates during the five last nuclear division cycles in the syncytial blastoderm, when multiple nuclei share the common cytoplasm.11 Activation of MAPK leads to the localized expression of the terminal gap genes, tailless (tll) and huckebein (hkb). This depends on the localized counteraction of transcriptional repression of tll and hkb by the HMG-box repressor Capicua (Cic) and the global co-repressor Groucho (Gro). Specifically, MAPK-mediated phosphorylation of Cic and Gro de-represses tll and hkb at the terminal regions of the embryo.12–15
The Torso pathway is arguably one of the best studied experimental systems for the in vivo analysis of RTK signaling. One of the main results is a gradient model of MAPK signaling and interpretation.16 The model can be summarized as follows: a combination of the uniformly expressed Torso and locally produced diffusible ligand generates a localized pattern of receptor occupancy on the surface of the embryo, which in turn generates a gradient of MAPK phosphorylation inside the embryo (Fig. 2A). The graded pattern of MAPK activation gives rise to the patterns of Cic and Gro modification and thus controls the expression of tll and hkb.
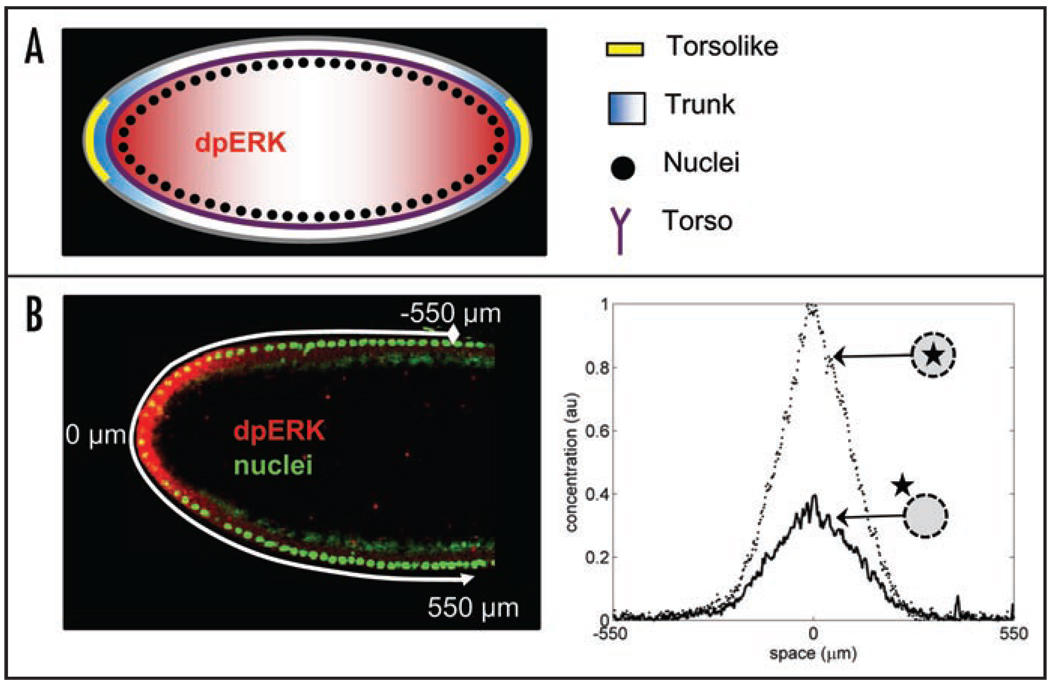
Figure 2.
(A) Torso receptors (purple) are uniformly distributed along the plasma membrane of the embryo. Inactive ligand (Trunk) is distributed uniformly in the extracellular matrix; it is converted into an active and diffusible form (blue) by Torsolike (yellow), a protein localized at the poles of the embryo.10 Torso-Trunk complex signals through the MAPK signaling cascade, which leads to MAPK phosphorylation. (B) Quantified pattern of MAPK phosphorylation. Left: fluorescent image of the anterior part of the embryo; nuclei are stained in green and phosphorylated MAPK is stained in red. Right: Gradients of nuclear and cytoplasmic phosphorylated MAPK. Reproduced from Berezhkovskii et al. PNAS 2009.
Notably, none of the spatial patterns involved in this model (those of ligand release, receptor occupancy, MAPK phosphorylation, etc.) have been characterized directly and most of the conclusions were derived from the analysis of the effects of various perturbations on either the morphology of the embryo or the expression of the terminal gap genes. To complement the genetic and biochemical approaches to the analysis of terminal system, we have recently developed a quantitative imaging assay of MAPK activation in the early embryo.17 Our assay relies on the previously developed antibody that recognizes the double phosphorylated form of ERK/MAPK (dpERK) and a suite of image processing tools for extracting and analyzing the spatial patterns of MAPK phosphorylation from a large collection of embryos.17,18
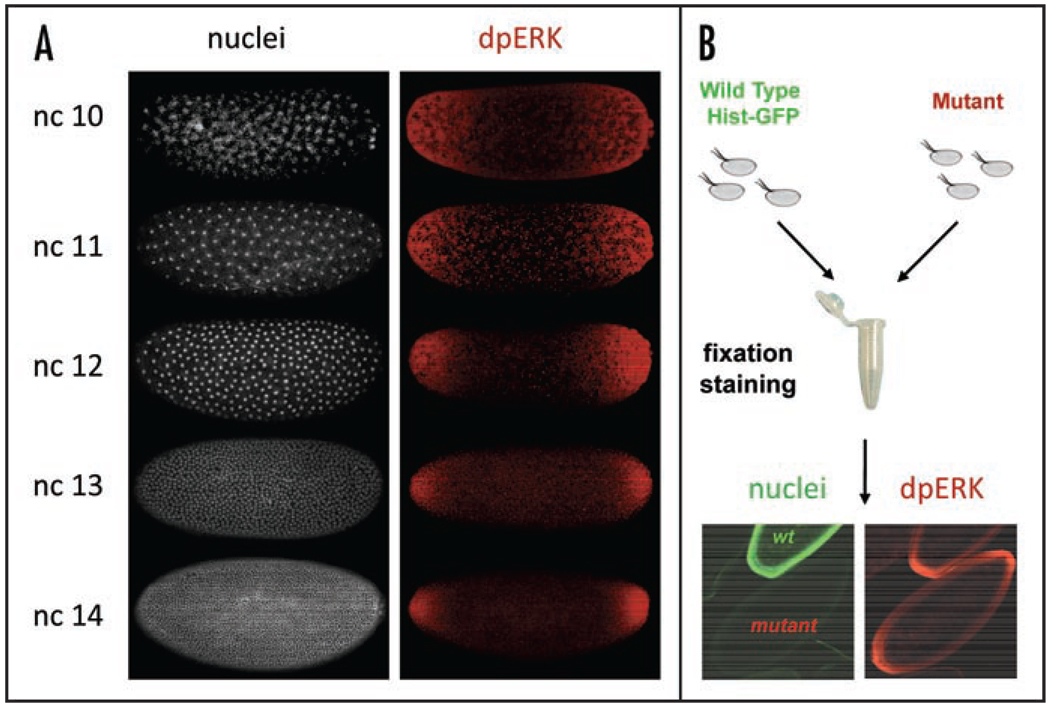
An example of a quantified MAPK phosphorylation pattern is shown in Figure 2B. Using embryos that carry a GFP-tagged histone, we can mark the nuclear compartment in the syncytium and thus “split” the gradient of MAPK phosphorylation into nuclear and cytoplasmic components. Furthermore, since the timings of nuclear divisions in the syncytial blastoderm have been measured rather precisely,11 nuclear density, which can be easily extracted from DNA stainings, provides at least five well-defined time points that span the time window during which the terminal system is activated. As a result, embryos stained for DNA and dpERK provide information about both the spatial and temporal aspects of MAPK phosphorylation (Fig. 3A). Finally, we have developed a straightforward technique for quantitative comparison of MAPK phosphorylation between the wild-type and any given mutant background (Fig. 3B).
Figure 3.
(A) The density of syncytial nuclei provides a time marker for MAPK phosphorylation at five distinct time points. (B) Summary of the experimental strategy for pairwise comparison of MAPK phosphorylation gradients between the wild-type and mutant backgrounds.
MAPK Activation by a Cascade of Diffusion Trapping Modules
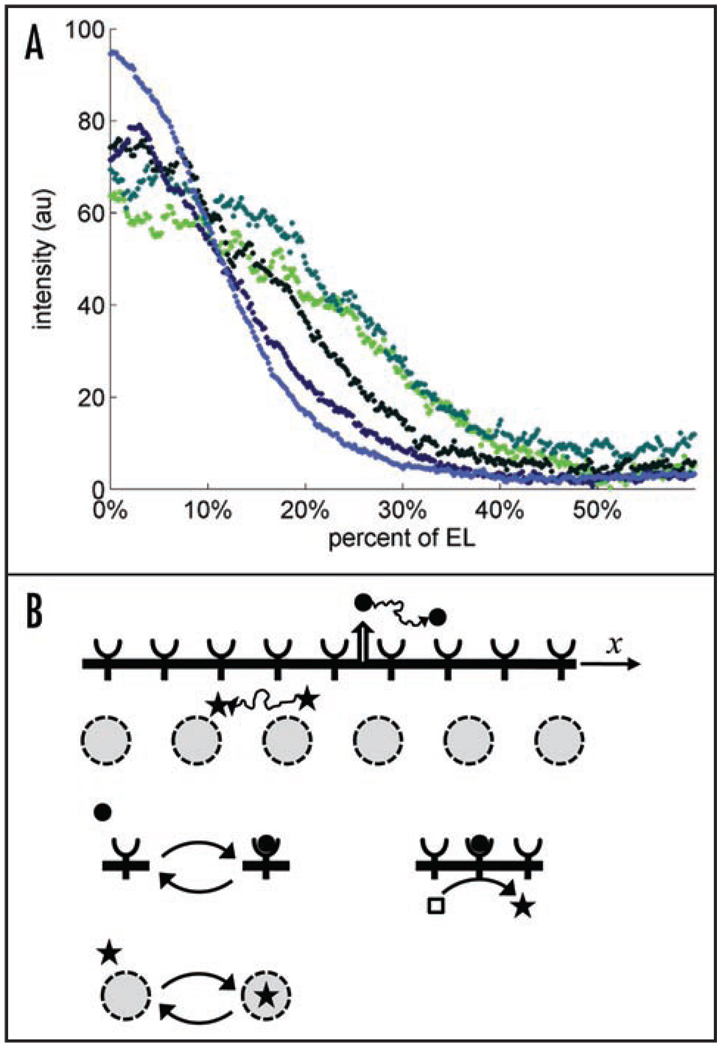
Using our quantitative imaging assay, we found that over the five last nuclear divisions, the level of MAPK phosphorylation is amplified at the poles and attenuated in the rest of the embryo (Fig. 4A). Nuclear divisions lead to an exponential increase in the number of nuclei per unit volume; hence, there is a clear correlation between the spatial pattern of MAPK activation and nuclear density. The observed dynamics are consistent with a model whereby dpERK is trapped by the nuclei: an increase in the nuclear density increases the trapping of phosphorylated MAPK and prevents its diffusion from the poles towards the middle of embryo (Fig. 4B).
Figure 4.
(A) Dynamics of the wild-type the MAPK phosphorylation gradient: over the time course of the five last nuclear divisions, the level of MAPK phosphorylation is amplified at both poles and attenuated in the rest of the embryo; reproduced from Coppey et al. Current Biology 2008; 18:915–9. These dynamics are consistent with a model based on a cascade of diffusion-trapping modules. (B) A diffusible ligand (solid circle) is reversibly bound by cell surface receptors. A diffusible intracellular molecule (star) shuttles in and out of the nuclei. Transitions between mobile and immobile states for a particle in the extracellular stage of the cascade. Immobile particles in the first stage initiate the production of mobile particles in the second stage. In this case, a ligand-receptor complex is an enzyme acting on a pool of inactive intracellular molecules (an empty square). A mobile intracellular molecule is reversibly trapped by immobile traps. In this case, the traps are nuclei distributed in a shared cytoplasm of the early embryo.
This model is based on the cell biological and biochemical studies that have established that phosphorylated MAPK rapidly translocates to the nucleus, which can also serve as a compartment of MAPK dephosphorylation.19–22 In combination with the progressive increase in nuclear density, these processes can amplify the dpERK levels at the poles and attenuate them in the rest of the embryo.23 Our subsequent experiments provided clear support for this model. In particular, we found pronounced disruptions in the spatial pattern of MAPK phosphorylation in embryos with defects in the spatial distribution of nuclei.17 Thus, syncytial nuclei not only sense the local level of MAPK phosphorylation that has been established by the upstream steps of MAPK activation, but actively control the spatial pattern of MAPK phosphorylation in the early embryo.
There is a strong biophysical analogy between this new intracellular diffusion-trapping module that controls the spatial distribution of phos-phorylated MAPK and the previously identified diffusion-trapping system that controls the spatial distribution of the extracellular ligand that activates Torso. Elegant genetic experiments by Casanova and Struhl established that removal of Torso receptors from the poles generates ectopic terminal structures in the middle of the embryo.24 Based on this, they proposed that uniformly expressed Torso both transduces the signal provided by Trunk and limits its diffusive spread. Indeed, ligand binding to Torso activates receptor tyrosine kinase signaling, but also leads to receptor-mediated ligand internalization.25 Casanova and Struhl have called this type of spatial regulation of receptor activation “ligand trapping”, an effect that has been subsequently identified in a large number of other patterning systems, both in Drosophila and other organisms.26,27
Since the spatial pattern of Torso occupancy on the plasma membrane provides an input that activates the MAPK cascade inside the embryo, it appears that the spatial pattern of MAPK activation is established by two sequentially acting diffusion-trapping systems. In the extracellular compartment, the Torso receptors limit the spatial spread of the Trunk ligand. Inside the embryo, nuclei limit the spread of diffusible dpERK. To assess the relative contributions of the extracellular and intracellular diffusion-trapping modules to establishing the final pattern of MAPK activation, we used a biophysical model for a cascade of diffusion trapping systems. A detailed biochemical model, like that discussed in the Introduction, contains a large number of species. Since only one of these species (dpERK) can be followed in the terminal system, we need the simplest possible model that is consistent with wild-type dynamics and can be used to predict the effects of genetic perturbations.
Biophysical Model
Our model for a cascade of two diffusion-trapping systems is given by the following equations:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Equation 1 and Equation 2 describe the concentrations of the extracellular ligand (active Trunk) and ligand-receptor complexes (Torso-Trunk) complexes, denoted by L(x,t) and C(x,t), respectively. Note that the receptor-bound ligand does not diffuse; this reflects the fact that the lateral movement of Torso receptors is highly restricted (personal communication).28 Equation 3 and Equation 4 describe the concentrations for the cytoplasmic and nuclear signaling molecules (phosphorylated MAPK), that are denoted by S(x,t) and N(x,t), respectively. Extracellular ligand is “injected” into the system at x = 0, with a time dependent flux, Q(t). A combination of ligand diffusion, reversible binding to surface receptors and receptor-mediated internalization establishes a spatial profile of cell surface receptor occupancy, which in turn serves as a source for the intracellular signaling species, which shuttle in and out of the nuclei and can be ‘degraded’ (dephosphorylated) in either of these compartments.
Equation 5 and Equation 6 provide the initial and boundary conditions. The system is modeled as one-dimensional (along the AP axis) and semi-infinite in space, reflecting the fact that the observed dpERK pattern is localized at the poles of the embryo.17 Receptors are assumed to be in excess and the rate constant of ligand binding, kb, is proportional to receptor expression level. Finally, all signal transduction processes, from the receptors to phosphorylated MAPK, have been lumped into a single constant, g, which characterizes the rate at which the dpERK molecules are generated (indirectly) by a single ligand/receptor complex.
The extracellular part of the model is essentially identical to those used to describe the patterning morphogens in other systems, e.g., the patterning of the follicular epithelium by Gurken or patterning of the wing imaginal disk by Dpp.29,30 The intracellular module is similar to the previously published descriptions of phosphorylation gradients in spatially distributed models of signal transduction pathways.31,32 Using this model we can explore how the pattern of MAPK phosphorylation is controlled by a combination of localized ligand release, extracellular diffusion, binding to cell surface receptors, receptor-mediated ligand internalization, and diffusion, nuclear trapping and dephosphorylation of activated MAPK inside the embryo.
Steady State Analysis of the Model
We analyzed our model in the regime where the level of ligand production and the properties of the syncytium are assumed to be constant (a blastoderm embryo at a given value of nuclear density). Quantitative analysis of the dpERK gradients in nuclear cycle 14 revealed that they exhibit very low variability.17 This suggests that the system is at steady state at a given setting of model parameters, such as receptor expression level and nuclear density. In this regime the spatial distributions of bound ligand and nuclear signal are given by the following expressions:
| (7) |
| (8) |
where λL and λS are the length scales of extracellular ligand and intracellular signal, respectively:
| (9) |
As in other models of diffusion and degradation, the length scale of a signaling molecule is an average distance to which it diffuses before being degraded.33,34
Analyzing equation 7 and equation 8 we found that the width of the cascade output, < x >output, depends on the relation between the length scales of signals in the extracellular and intracellular compartments. Specifically, when λL/λS ≫ 1, the spatial distribution of the output is controlled by extracellular module. In the opposite extreme, when λL/λS ≪ 1, < x >output is controlled by the intracellular module: < x >output ≈ λS, which would imply that the pattern of receptor occupancy is sharply localized and the shape of the output is determined by processes inside the embryo.
These two limiting regimes can be distinguished by their responses to changes in the length scale of individual stages. In particular, if λL/λS ≪ 1, then the spatial profile of the output should be insensitive to further reduction in the length scale of the extracellular module. Genetically, λL can be manipulated by changing the level of Torso receptors, which bind and internalize the diffusible ligand. In particular, λL should be increased/decreased by reductions/increases in the copy number of Torso.
Using our approach for pairwise comparison of dpERK gradients in mutant genetic backgrounds, we found that the spatial patterns of MAPK phosphorylation in embryos with only one copy of Torso and embryos with two extra copies Torso are essentially indistinguishable from those in the wild-type embryos.17 Thus, the terminal system operates in the regime where λL/λS ≪ 1. At the same time, increases in the nuclear density because of the nuclear divisions should decrease the value of λS. The fact that the wild-type patterns of MAPK phosphorylation are sharpened in response to increases in the nuclear density is consistent with our hypothesis that nuclei play an active role in shaping the spatial pattern of MAPK phosphorylation.
Summary and Outlook
Depending on the cellular context, the MAPK cascade can act as a graded or ultrasensitive switch, and, when embedded in networks with feedbacks, lead to bistable and oscillatory dynamics.8,35–40 Thus, the rigid structure of the cascade does not imply a rigid function, as was proposed by the early mathematical models of this pathway, and can instead lead to a very diverse range of input-output maps. Given a large number of the pathway components and modes of regulation, it is essential to establish experimental systems that would allow both the manipulation of the MAPK cascade and monitoring its dynamics. The terminal patterning system appears to be ideally suited for this purpose because of its relative anatomical simplicity and the availability of a large number of genetic tools for cascade manipulation.
Our recent results provided the first direct measurements of the MAPK phosphorylation gradient in the early embryo.17 We characterized the dynamics of the gradient in vivo and proposed that it is regulated by the nuclear trapping of phosphorylated MAPK. This new type of information (quantitative characteristics of the gradient vs. qualitative data from genetic experiments) requires a new type of language for analyzing and conceptualizing the data. We have begun to develop biophysical models that can potentially provide such a language and guide experiments for exploring the relative importance of multiple layers of regulation.23
Several aspects of our nuclear trapping model for the spatial regulation of MAPK phosphorylation in the syncytium require further investigation. One of the most important outstanding questions is the identity and subcellular localization of MAPK phosphatases.21,22 A number of candidate phosphatases exist in the genome and their individual contributions can be assessed using our quantitative imaging approach, by comparing the patterns of MAPK phosphorylation between the wild-type and single mutant backgrounds. Finally, all of our results relied on experiments that followed a single component (dpERK) and fixed samples. One can only imagine the wealth of information and new ideas that will be derived from experiments that can track multiple pathway components in live embryos.19
Acknowledgements
We thank Alistair Boettiger, Qiao Liang and Yannis Kevrekidis for numerous helpful discussions. This work was supported by the Intramural Research Program of the NIH, Center for Information Technology (Alexander M. Berezhkovskii), by the P50 GM071508 and R01 RM078079 grants from the NIH (Stanislav Y. Shvartsman), and by the NIH Contract No.HHSN266200500021C, ADB No. N01-AI-50021 (Stanislav Y. Shvartsman and Mathieu Coppey).
References
- 1.Chang LF, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu BE, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Arias A, Stewart A. Molecular principles of animal development. New York: Oxford University Press; 2002. [Google Scholar]
- 4.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Hornberg JJ, Binder B, Bruggeman FJ, Schoeberl B, Heinrich R, Westerhoff HV. Control of MAPK signalling: from complexity to what really matters. Oncogene. 2005;24:5533–5542. doi: 10.1038/sj.onc.1208817. [DOI] [PubMed] [Google Scholar]
- 6.Kholodenko BN. Cell-signalling dynamics in time and space. Nat Rev Mol Cell Biol. 2006;7:165–176. doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blüthgen N, Legewie S. Systems analysis of MAPK signal transduction. Essays Biochem. 2008:45. doi: 10.1042/BSE0450095. [DOI] [PubMed] [Google Scholar]
- 8.Huang C, Ferrell JJ. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiao L, Nachbar RB, Kevrekidis IG, Shvartsman SY. Bistability and oscillations in the Huang-Ferrell model of MAPK signaling. PLoS Comput Biol. 2007;3:1819–1826. doi: 10.1371/journal.pcbi.0030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li WX. Functions and mechanisms of receptor tyrosine kinase Torso signaling: lessons from Drosophila embryonic terminal development. Dev Dyn. 2005;232:656–672. doi: 10.1002/dvdy.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behavior during the 5 mitotic-cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez G, Guichet A, Ephrussi A, Casanova J. Relief of gene repression by Torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes Dev. 2000;14:224–231. [PMC free article] [PubMed] [Google Scholar]
- 13.Paroush Z, Wainwright SM, IshHorowicz D. Torso signalling regulates terminal patterning in Drosophila by antagonising Groucho-mediated repression. Development. 1997;124:3827–3834. doi: 10.1242/dev.124.19.3827. [DOI] [PubMed] [Google Scholar]
- 14.Astigarraga S, Grossman R, Diaz-Delfin J, Caelles C, Paroush Z, Jimenez G. A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. EMBO J. 2007;26:668–677. doi: 10.1038/sj.emboj.7601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cinnamon E, Helman A, Ben-Haroush SR, Orian A, Jiménez G, Paroush Z. Multiple RTK pathways downregulate Groucho-mediated repression in Drosophila embryogenesis. Development. 2008;135:829–837. doi: 10.1242/dev.015206. [DOI] [PubMed] [Google Scholar]
- 16.Furriols M, Casanova J. In and out of Torso RTK signalling. EMBO J. 2003;22:1947–1952. doi: 10.1093/emboj/cdg224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppey M, Boettiger AN, Berezhkovskii AM, Shvartsman SY. Nuclear trapping shapes the terminal gradient in the Drosophila embryo. Curr Biol. 2008;18:915–919. doi: 10.1016/j.cub.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabay L, Seger R, Shilo BZ. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development. 1997;124:3535–3541. doi: 10.1242/dev.124.18.3535. [DOI] [PubMed] [Google Scholar]
- 19.Fujioka A, Terai K, Itoh RE, Aoki K, Nakamura T, Kuroda S, Nishida E, Matsuda M. Dynamics of the Ras/ERK MAPK cascade as monitored by fluorescent probes. J Biol Chem. 2006;281:8917–8926. doi: 10.1074/jbc.M509344200. [DOI] [PubMed] [Google Scholar]
- 20.Costa M, Marchi M, Cardarelli F, Roy A, Beltram F, Maffei L, Ratto GM. Dynamic regulation of ERK2 nuclear translocation and mobility in living cells. J Cell Sci. 2006;119:4952–4963. doi: 10.1242/jcs.03272. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson M, Mandl M, Keyse SM. Spatio-temporal regulation of mitogen-activated protein kinase (MAPK) signalling by protein phosphatases. Biochem Soc Trans. 2006;34:842–845. doi: 10.1042/BST0340842. [DOI] [PubMed] [Google Scholar]
- 22.Caunt CJ, Armstrong SP, Rivers CA, Norman MR, McArdle CA. Spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem. 2008;283:26612–26623. doi: 10.1074/jbc.M801500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berezhkovskii AM, Coppey M, Shvartsman SY. Signaling gradients in cascades of two-state reaction-diffusion systems. Proc Natl Acad Sci. 2009;106:1087–1092. doi: 10.1073/pnas.0811807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casanova J, Struhl G. The torso receptor localizes as well as transduces the spatial signal specifying terminal body pattern in Drosophila. Nature. 1993;362:152–155. doi: 10.1038/362152a0. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 27.Scholpp S, Brand M. Endocytosis controls spreading and effective signaling range of Fgf8 protein. Curr Biol. 2004;14:1834–1841. doi: 10.1016/j.cub.2004.09.084. [DOI] [PubMed] [Google Scholar]
- 28.Mavrakis M, Rikhy R, Lippincott-Schwartz J. Plasma membrane polarity and compartmentalization are established prior to cellularization in the fly embryo. Dev Cell. 2009;16:93–104. doi: 10.1016/j.devcel.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruse K, Pantazis P, Bollenbach T, Julicher F, Gonzalez-Gaitan M. Dpp gradient formation by dynamin-dependent endocytosis: receptor trafficking and the diffusion model. Development. 2004;131:4843–4856. doi: 10.1242/dev.01335. [DOI] [PubMed] [Google Scholar]
- 30.Goentoro LA, Reeves GT, Kowal CP, Martinelli L, Schupbach T, Shvartsman SY. Quantifying the gurken morphogen gradient in Drosophila oogenesis. Dev Cell. 2006;11:263–272. doi: 10.1016/j.devcel.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Albada SB, ten Wolde PR. Enzyme localization can drastically affect signal amplification in signal transduction pathways. PLoS Comput Biol. 2007;3:1925–1934. doi: 10.1371/journal.pcbi.0030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kholodenko BN, Brown GC, Hoek JB. Diffusion control of protein phosphorylation in signal transduction pathways. Biochem J. 2000;350:901–907. [PMC free article] [PubMed] [Google Scholar]
- 33.Kicheva A, Pantazis P, Bollenbach T, Kalaidzidis Y, Bittig T, Jülicher F, González-Gaitán M. Kinetics of morphogen gradient formation. Science. 2007;315:521–525. doi: 10.1126/science.1135774. [DOI] [PubMed] [Google Scholar]
- 34.Reeves GT, Muratov CB, Schupbach T, Shvartsman SY. Quantitative models of developmental pattern formation. Dev Cell. 2006;11:289–300. doi: 10.1016/j.devcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Mackeigan JP, Murphy LO, Dimitri CA, Blenis J. Graded mitogen-activated protein kinase activity precedes switch-like c-Fos induction in mammalian cells. Mol Cell Biol. 2005;25:4676–4682. doi: 10.1128/MCB.25.11.4676-4682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong W, Ferrell JEJ. A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 37.Kholodenko B. Negative feedback and ultrasensitivity can bring about oscillations in the mitogen-activated protein kinase cascades. Eur J Biochem. 2000;267:1583–1588. doi: 10.1046/j.1432-1327.2000.01197.x. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama K, Satoh T, Igari A, Kageyama R, Nishida E. FGF induces oscillations of Hes1 expression and Ras/ERK activation. Curr Biol. 2008;18:332–334. doi: 10.1016/j.cub.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Hilioti Z, Sabbagh WJ, Paliwal S, Bergmann A, Goncalves MD, Bardwell L, Levchenko A. Oscillatory phosphorylation of yeast Fus3 MAP kinase controls periodic gene expression and morphogenesis. Curr Biol. 2008;18:1700–1706. doi: 10.1016/j.cub.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi S, Pryciak PM. Membrane localization of scaffold proteins promotes graded signaling in the yeast MAP kinase cascade. Curr Biol. 2008;18:1184–1191. doi: 10.1016/j.cub.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]