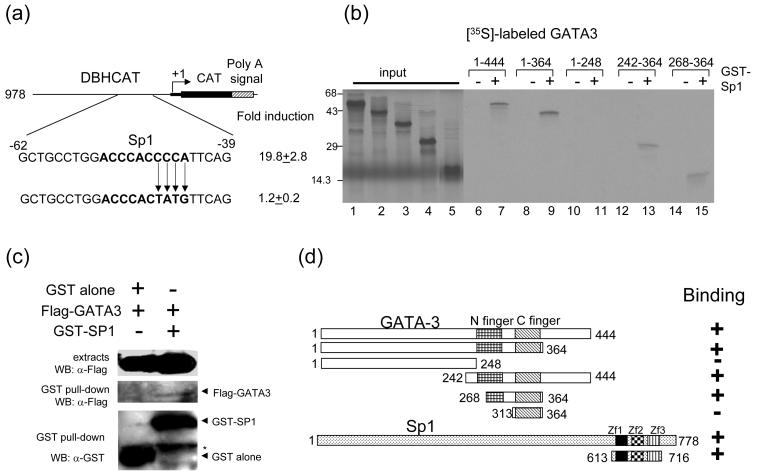
Fig. 3.
GATA-3 physically interacts with Sp1. (a) Mutation of Sp1 binding site in DBH978CAT diminishes reporter gene activation by GATA-3. The bold characters represent the Sp1 binding site. (b) Localization of the interaction domains of GATA-3 and Sp1. In vitro translated [35S]-methionine labeled GATA-3 proteins were incubated with GST (lanes 6, 8, 10, 12, and 14) or with full-length GST-Sp1 (lanes 7, 9, 11, 13, and 15) bound to glutathione Sepharose beads. [35S]-methionine labeled input proteins are shown (lanes 1 (aa 1-144), 2 (aa 1-364), 3 (aa 1-248), 4 (aa 242-364) and 5 (aa 268-364)). The numbers at the top of the figure represent the amino acid residues of GATA3. (c) Interaction between GATA-3 and Sp1 in vivo. 293T cells were cotransfected with the Sp1 (aa 613 - 716), and empty vector or GATA-3 as indicated on the top. The cell lysates were precipitated with glutathione-Sepharose beads. Monoclonal anti-FLAG was used to detect the FLAG-tagged GATA-3 proteins. The blot was stripped and re-probed with the anti-GST to detect the precipitated Sp1proteins (bottom). Top, crude cell extracts; middle, immunoprecipitates; bottom, GST-Sp1. Asterisk indicates non-specific bands containing the heavy chain of IgG. (d) Summary of the interaction results of GATA3 with Sp1. Three zinc finger motifs (Zf1-3) of Sp1 are indicated.