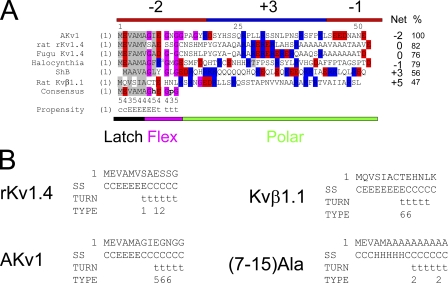
Figure 2.
Homology relationships between different Shaker channel N-type inactivation domains. (A) Alignments suggest the partitioning of N terminus into three distinct regions: a hydrophobic latch region, a glycine-rich flex region, and a charged polar region. Specific sequence conservation between N termini is only seen in the latch and flex regions (see Consensus; h, a hydrophobic residue; p, a polar residue). The number below the consensus indicates the number of the six sequences that matches the consensus. Percent identity with consensus ranges from 100% for AKv1 to 47% for Kvβ1.1 (%). Structural prediction for the consensus is given: C, coil; E, extended; t, β turn. Although polar region sequence conservation is low, there is a general charge trend, where this region is predominantly positively charged close to the N terminus but becomes more negative near the T1 domain at the C terminus. Positive charges are not typically found in the latch and flex regions, which typically have a net negative charge. Net charge of N termini varies widely from +5 to −2; however, the general pattern of a more positive N-terminal part of the polar region remains. (B) Structural predictions for selected N termini. General trend for a latch region extended structure and a flex region β turn is present in all N termini but (7–15)Ala, which predicts an α helix at the beginning of the flex region. Turn type code: 1, type I; 2, type II; 3, type VIII; 4, type I' ; 5, type II'; 6, type IV (Fuchs and Alix, 2005).