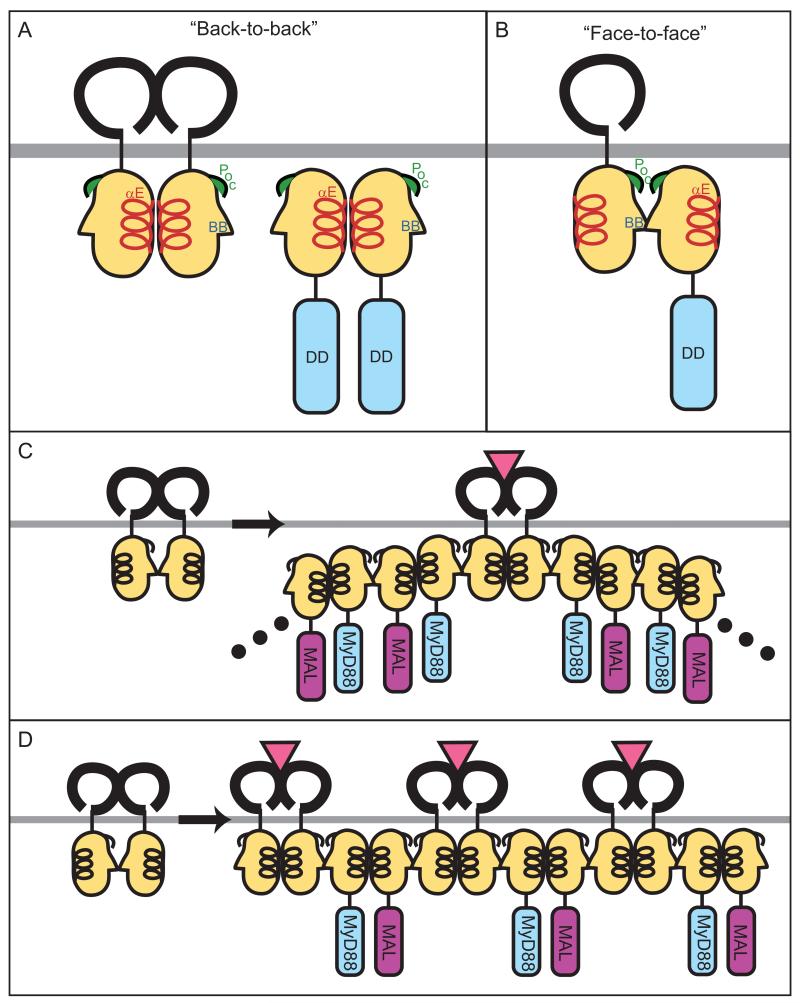
Fig. 1. TLR:MyD88 interactions.
The interfaces between two receptors or two adapters or receptor and adapter are predicted from docking studies based on the structures of the TIR domains of TLR1 and TLR2. (A) Binding between two receptors or two adapters may be mediated by ‘back-to-back’ TIR domain (light orange) interactions, which are characterized by homotypic interactions between αE helices (red). The back-to-back interface does not mediate receptor:adapter binding. (B) ‘Face-to-face’ TIR domain interactions likely mediate receptor:adapter binding and involve the BB loop (blue) and Poc site (green) from each molecule. Whether face-to-face interactions can also mediate receptor:receptor or adapter:adapter binding is untested but expected based on molecular docking studies. (C and D) Models for recruitment and ‘chaining’ of adapter molecules upon TLR activation. In (C), ligand binding causes dimerization of two TLRs in a back-to-back fashion, followed by recruitment of adapter (MyD88) to the receptor by a face-to-face interaction. Signal amplification may be achieved by the subsequent recruitment of additional adapters (MyD88 and MAL), which bind each other with alternating face-to-face and back-to-back interactions. Alternatively, as shown in (D), upon ligand binding and back-to-back dimerization of receptors, each receptor complex recruits one adapter by a face-to-face interaction. Adjacent adapters (MyD88 and MAL) then form back-to-back interfaces.