Abstract
The Leishmania spp. protozoa have an abundant surface metalloprotease called MSP (major surface protease), which in Leishmania chagasi is encoded by three distinct gene classes (MSPS, MSPL, MSPC). Although MSP has been characterized primarily in extracellular promastigotes, it also facilitates survival of intracellular amastigotes. Promastigotes express MSPS and MSPL RNAs, and two forms of MSPC RNA, whereas amastigotes express only MSPL RNA and one MSPC transcript. We confirmed the presence of MSPC protein in both promastigotes and amastigotes by Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS). More than 10 MSP isoforms were visualized in both amastigotes and promastigotes using two-dimensional immunoblots, but amastigote MSPs migrated at a more acidic pI. Promastigote MSPs were N-glycosylated, whereas most amastigote MSPs were not. Immuno-electron microscopy showed that two-thirds of the promastigote MSP is distributed along the cell surface. In contrast, most amastigote MSP localized at the flagellar pocket, the major site of leishmania endocytosis/exocytosis. Biochemical analyses indicated that most amastigote MSP is soluble in the cytosol, vesicles or organelles, whereas most promastigote MSP is membrane-associated and GPI anchored. Activity gels and immunoblots confirmed the presence of a novel proteolytically active amastigote MSP of higher Mr than the promastigote MSPs. Furthermore, promastigote MSP is shed extracellularly whereas MSP is not shed from axenic amastigotes. We conclude that amastigote and promastigotes both express multiple MSP isoforms, but these MSPs differ biochemically and localize differently between the parasite stages. We hypothesize that MSP plays different roles in the extracellular versus intracellular forms of Leishmania spp.
Keywords: Leishmania, protease, amastigote, metalloprotease, flagellar pocket
1. Introduction
MSP (also called GP63 or leishmanolysin) is an abundant metalloprotease on the surface membrane of all Leishmania spp. studied to date [1, 2]. L. chagasi has at least eighteen MSP genes located in a tandem array on a single chromosome [3, 4]. We previously showed that the increased abundance of MSP in some forms of L. chagasi promastigotes correlates with increased parasite virulence [5, 6]
Leishmania spp. have a dimorphic life cycle. The extracellular flagellated promastigote develops to an infectious form in the gut of the sand fly vector. After inoculation into mammalian skin during a sand fly blood meal, the promastigote is engulfed by local macrophages and transforms into the intracellular amastigote stage [1, 7]. MSP is highly abundant in virulent promastigotes, comprising 1% of the total protein [8]. Eleven isoforms of MSP protein are detected in virulent promastigotes of L. chagasi by two-dimensional gel profiles [9, 10]. Although MSP is not well characterized in intracellular amastigotes, it is also expressed in these cells [11, 12]. The purpose of this study was to compare and contrast the biochemical and cellular properties of MSP in L. chagasi promastigotes and amastigotes.
L. chagasi has three classes of MSP genes, called MSPL, MSPS and MSPC [6]. As indicated by their deduced amino acid sequences, MSPL and MSPS both contain a glycosylphosphatidylinositol (GPI) attachment site near their C termini, whereas MSPC encodes a protein apparently lacking the typical GPI anchor addition site [6]. MSP is a zinc metalloprotease active against a wide spectrum of substrates which plays a vital role in leishmania pathogenesis. MSP has been shown to (i) facilitate promastigote evasion of complement-mediated lysis, (ii) promote attachment of promastigotes to macrophage complement receptors, (iii) enhance the intracellular survival of amastigotes, and (iv) degrade some host cytosolic proteins [13–20].
Biochemical studies of intracellular Leishmania spp. amastigotes have been hampered by contamination of animal-derived or macrophage-derived amastigote preparations with host macrophage proteins. As such, investigators have developed axenic systems for cultivating amastigotes in vitro, using conditions designed to simulate the mammalian tissue environment (elevated temperature and decreased pH). Unfortunately not all Leishmania species readily convert to axenic amastigotes in vitro [21–25]. By modifying a published in vitro differentiation protocol [23], we were able to generate a line of L. chagasi, called LcJ, that converts back and forth between promastigotes and amastigotes in vitro. Using the LcJ line, we examined MSP expression in L. chagasi amastigotes and promastigotes. In contrast to long-term cultured promastigotes which lose the ability to express MSP, LcJ promastigotes retain their expression of surface MSP if they are passed alternately as promastigotes and amastigotes. We found differences in the biochemical modifications of the MSP gene products, and differences in the predominant genes expressed. Studies of MSP intracellular localization and release into the extracellular environment suggest there are major differences in the function(s) of this protease in the intracellular and extracellular stages of Leishmania species.
2. Materials and methods
2.1. Parasites and Bacteria
A Brazilian strain of L. chagasi (MHOM/BR/00/1669) was originally isolated from a patient with visceral leishmaniasis. Parasites were passed through male golden hamsters to maintain their virulence, and used within 3 weeks of isolation from the animal. Promastigotes were cultured at 26°C in hemoflagellate-modified minimal essential medium (HOMEM), pH 7.4, supplemented with 10 % heat-inactivated fetal calf serum [26]. Amastigotes were cultivated in medium containing 20% FCS at 37°C, 5% CO2, pH 5.5, as previously described for L. donovani [25]. The stage-converting strain LcJ was derived from wild type L. chagasi by cultivation in amastigote medium. LcJ amastigotes were passed at least once in amastigote growth conditions to ensure full stage conversion prior to all the experiments except for the immunoblot study of MSP during stage conversion (Figure 1). LcJ promastigotes did not differ in cell morphology from wild type L. chagasi. Logarithmic and stationary phase promastigote populations were previously defined by cell density and morphology [27].
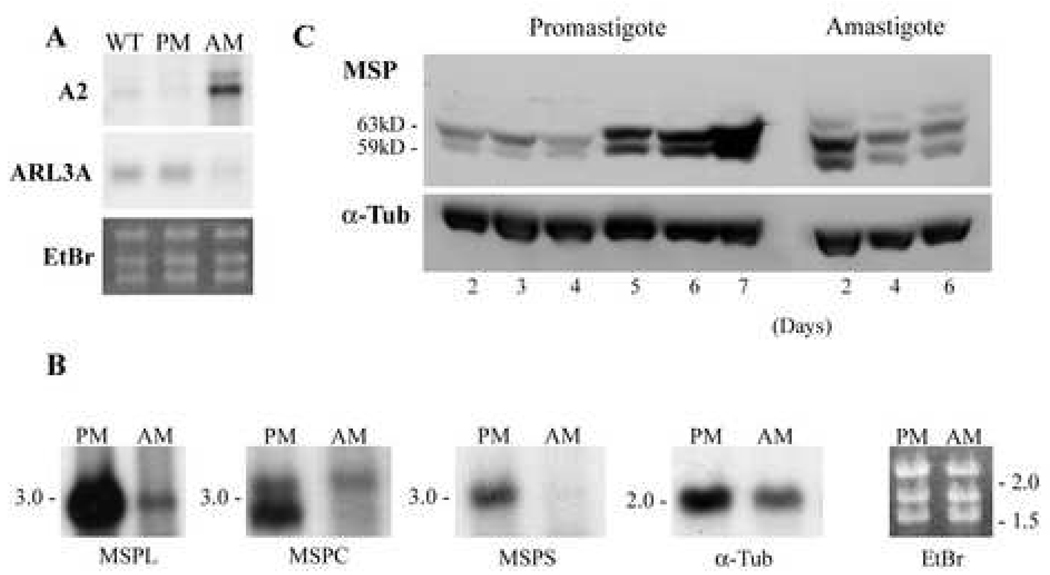
Fig. 1.
Differential expression of RNAs by promastigotes and axenic amastigotes, and of MSP protein during successive days of cultivation in vitro. A and B. Northern blots. A. Total RNA was extracted from wild type L. chagasi promastigotes (WT), LcJ promastigotes (PM), or LcJ axenic amastigotes (AM). RNA was separated on 1.2% agarose gels and hybridized with [32P]-DNA probes corresponding to the indicated genes. B. Total RNAs from LcJ promastigotes (PM) or axenic LcJ amastigotes (AM) were probed in northern blots with a [32P]-DNAs containing the unique 3’ UTR sequences of the MSPL, MSPC and MSPS genes. Blots were reprobed with the coding sequence for α-tubulin. The last panel shows a representative ethidium-bromide (EtBr) stain documenting equal loading of lanes. Numbers on the left of the panels indicate the sizes in kb of marker RNAs. C. Western blots. Total lysates of LcJ promastigotes were harvested on days 2–7 of growth. Promastigotes were suspended in differentiation medium to promote conversion to axenic amastigotes, and amastigote protein lysates were harvested after 2, 4, or 6 days. Lysates of 5 × 106 promastigotes or 1 × 107 amastigotes were separated on 7.5% gels and MSP was identified on an immunoblot probed with sheep polyclonal antiserum against MSP proteins. Blots were stripped and incubated with antibody to α-tubulin as a loading control.
Avirulent Salmonella typhimurium, BJ2565 strain, a kind gift from Dr. Bradley Jones at the University of Iowa, was grown in Luria Broth containing 50 µg kanamycin/ml. This strain lacks the upstream repressing sequence in the hilA promoter that controls the expression of hilA, a transcriptional regulator to Salmonella pathogenicity island 1 (SPI-1) invasion genes [28].
2.2. Antibodies
Antiserum to MSP was raised in sheep against purified L. chagasi promastigote MSP as we previously reported [26]. For electron microscopy, sheep anti-MSP was affinity purified [29]. Briefly, L. chagasi promastigote membrane MSP was purified with Triton X-114 (TX-114), separated on 7.5% SDS polyacrylamide gels, and transferred to PVDF membranes. Protein was eluted with 50 mM glycine, pH 2.5 at 4°C in minimal volume. CA7AE antisera specific for unsubstituted phosphoglycan repeats was used for immunoblots [30]. Monoclonal antibody to chicken α-tubulin was purchased from CalbioChem (San Diego, CA). Peroxidase-conjugated anti-sheep and anti-mouse sera were purchased from Kierkegaard & Perry Laboratories (Gaithersburg, MA) and Bio-Rad Laboratories (Richmond, CA), respectively.
2.3. One- and two-dimensional electrophoresis and immunoblots
Lysates of promastigotes or amastigotes were separated on 7.5% polyacrylamide gels, transferred to nitrocellulose membranes (Schleicher & Schuell Bioscience, Keene, NH), blocked in 5% dry milk, 0.1%Tween 20 in phosphate buffered saline (PBS) and incubated with antibodies [10]. Primary and secondary antibodies were diluted 1:10,000 unless otherwise specified. Blots were developed by enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech, Arlington Heights, IL).
Parasite proteins were prepared in isoelectric focusing (IEF) lysis buffer (9M urea, 2% Triton X-100, 1% DTT, 0.8% carrier ampholytes) for two-dimensional electrophoresis. Proteins were separated according to their isoelectric points (pI) on Immobiline™ DryStrip pH 4–7 hydrated in 8 M urea, 0.5%Triton X-100, 0.2% DTT, and 0.2% carrier ampholytes (Amersham Biosciences, Piscataway, NJ). Gel strips were overlaid on 7.5% SDS polyacrylamide gels and proteins were separated by mass in the second dimension. Protein concentrations were determined by BCA assay (PIERCE, Rockford, IL).
Two-dimensional gels were either silver stained using SilverQuest™ Staining Kit (Invitrogen, Carlsbad, CA) or used for immunoblots as described for one-dimensional blotting.
2.4. Northern blot
Total RNA was extracted from promastigotes or amastigotes by the method of Chomczynski [31]. Four µg of RNA were separated in 1.2% formaldehyde-containing agarose gels and transferred by capillary action to nylon filters (Roche, Germany). The blots were probed with [32P]-DNA corresponding to the amastigote specific gene A2, the unique 3’ untranslated regions (UTRs) of the MSPL, MSPS and MSPC genes [4, 6], or the coding region of α-tubulin, amplified by the polymerase chain reaction from L. chagasi genomic DNA. The blots were washed at high stringency (0.1X SSC, 0.1% SDS, 65°C) and exposed to X-ray film.
2.5. Triton X-114 partial purification of MSP
MSP and other membrane proteins were extracted from 1 × 109 LcJ or wild type L. chagasi promastigotes, or from LcJ amastigotes with 1% TX-114 at 4°C. Due to the low cloud point of TX-114 (20°C) amphipathic (detergent phase) molecules were separated from hydrophilic (aqueous phase) molecules by heating to 37°C and microcentrifugation through a 6% sucrose cushion at room temperature [8, 32]. Partitions were repeated twice to purify proteins.
2.6. Glycosidase treatment
3 × 107 stationary promastigotes and fully converted amastigotes (day 10–12 after stage conversion) were lysed in denaturing buffer (2.5% SDS, 5% β-mercaptoethanol, 100°C, 10 min). Samples were added to adjust to 0.05 M sodium phosphate, pH 7.5, 1% NP40, and then treated with 500 Units Peptide N-Glycosidase F (PNGase F, New England Biolabs Inc., Beverley, MA) for 3 hours at 37°C. PNGase F specifically removes N-linked glycoproteins. Cleavage was documented by increased migration through polyacrylamide gels using immunoblots.
2.7. Electron microscopy
Stationary phase LcJ promastigotes, LcJ amastigotes, or S. typhimurium were fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in PBS overnight at 4°C, dehydrated in a series of ethanol buffers, and embedded in LR White resin (Structure Probe, Inc./SPI Supplies, West Chester, PA). Ultrathin sections on nickel grids were incubated with affinity purified sheep anti-MSP sera (1:400 dilution) followed by rabbit anti-sheep antibodies conjugated to ultra small gold particles (<0.8nm; 1:50 dilution, Electron Microscopy Sciences, Hatfield, PA) enhanced with silver. Samples were post-fixed with 2.5% glutaraldehyde, counterstained with uranyl acetate and lead citrate, and examined by JEOL 123 and Hitachi H-7000 transmission electron microscopes. Procedures were preformed in the Central Microscopy Facility, University of Iowa.
2.8. Subcellular fractionation
LcJ amastigotes and stationary phase growth phase promastigotes were washed twice and resuspended at 2 × 108/ml in hypotonic buffer (1mM potassium acetate, 1.5 mM magnesium acetate, 1 mM CaCl2, 10 mM Tris, 2 mM EDTA, pH 7.2) containing DNase I, RNase A and protease inhibitor cocktail set IV (Calbiochem, San Diego, CA). After incubation on ice for 25 min, the lysate was subjected to six freeze-thaw cycles and centrifugation at 5000 g for 15 min to remove non-lysed parasites. The supernatant fraction containing the parasite lysate was subjected to centrifugation at 100,000 g for 1 hr using a Beckman TLS55 rotor. The resultant supernatant containing cytosolic components (S1) was removed and the resultant pellet (P1) containing microsomes (membranes and organelles) was subjected to one of the following treatments. In the first treatment, pellet P1 was resuspended in SDS loading buffer. In the second, P1 was washed with 100 mM sodium carbonate (pH11.5). In the third, P1 was washed with 1 M potassium chloride. Washed samples were incubated on ice for 30 min followed by 100,000 g centrifugation as above.
2.9. Protease activity gel
Amastigotes or promastigotes were collected and lysed in non-reducing SDS-PAGE loading buffer without β-mercaptoethanol or heating. Samples were separated in a 7.5% gel containing 0.2% gelatin. 2 × 107 cell equivalents of stationary phase promastigotes or 5 × 107 cell equivalents of amastigotes were loaded on the gel, respectively. After electrophoresis, gels were equilibrated in three changes (30 min each) in either TBS buffer (10 mM Tris and 150 mM NaCl, pH 7.9) or Britton-Robinson type universal buffer (31 mM citric acid, 29 mM each of KH2PO4, boric acid and Tris/HCl, pH 5.5) [33]. 2.5% Triton X-100 was added in the first incubation to remove SDS. Gels were subsequently incubated in the same fresh buffer at 37°C for 24 hours in the presence or absence of 10mM 1,10-phenanthroline to inhibit metalloprotease activity. The gels were stained with Coomassie Blue. Gelatinolytic activity of proteases appeared as clear areas in the blue background. Clear bands were cut out of some gels, boiled in SDS reducing buffer, and analyzed by western blotting with polyclonal anti-MSP.
3. Results
3.1. Axenic amastigotes
Upon incubation in amastigote medium (pH 5.5) at 37°C, the elongated flagellated stationary phase LcJ line L. chagasi promastigotes transformed within 24 to 48 hours to aflagellate amastigotes, which were able to replicate continuously in culture. The similarity of axenic LcJ amastigotes to true amastigotes was documented by probing northern blots of total RNAs from wild type promastigotes, LcJ promastigotes or LcJ amastigotes with [32P]-DNA sequences of the A2 gene, which is expressed only in amastigotes [34], or the ARL3A gene, which is expressed predominately in promastigotes [35] [Figure 1A]. These blots showed high expression of A2 RNA in amastigotes but not promastigotes, and more abundant expression of ARL3A in both wild type and LcJ promastigotes than in LcJ amastigotes. As additional validation, we performed immunoblots with CA7AE antibody to phosphoglycan repeats, and showed that wild type L. chagasi and LcJ promastigotes contain lipophosphoglycan (LPG) and high Mr proteophosphoglycans (PPGs), whereas LcJ amastigotes contain PPGs but not LPG (data not shown).
3.2. MSP expression in life stages of L. chagasi
Another validation that axenic amastigotes are a reasonable model of true amastigotes was derived from their expression of MSP gene classes on northern blots (Figure 1B). Transcripts of the three MSP gene classes are differentially expressed during development of L. chagasi promastigotes in vitro from less virulent logarithmic to more virulent stationary phase promastigotes [6]. Amastigotes were found to express MSPL and MSPC transcripts but lack detectable MSPS RNA (Figure 1B). Furthermore, amastigotes contained only the 3.1 kb form of MSPC, in contrast to promastigotes which contain both 2.6 and 3.1.kb forms of MSPC RNA [6]. These data are consistent with our previous observations using cell-line grown amastigotes [36]. These earlier studies indicated that the two sizes of MSPC RNA result from the use of different poly(A) addition sites [36], and suggest there may be differences between amastigote and promastigote post-transcriptional modifications. The ethidium bromide stain and a reprobe of the northern blot with the α-tubulin coding region demonstrated comparable loading of promastigote and amastigote lanes. Thus, MSP gene expression by axenic LcJ amastigotes is similar to that of true amastigotes. We also attempted northern blots of hamster tissue-derived amastigote RNA. Similar to our prior experience, we found that transcripts from tissue derived amastigotes degraded rapidly and could not be assessed reliably by northern blots.
The abundance of MSP proteins increases 14-fold as virulent L. chagasi promastigotes grow from logarithmic to stationary phase in liquid culture. There is not a corresponding change in the steady state abundance of total MSP RNA [15]. Promastigotes also express several MSP protein isoforms, and in particular a 59-kDa MSP isoform correlates with parasite virulence [26]. To discern the MSP isoforms expressed by amastigotes, we performed immunoblots on one-dimensional gels using lysates of LcJ promastigotes or LcJ amastigotes (Figure 1C). MSP abundance increased in LcJ promastigotes as they progressed from logarithmic (days 2–3) to stationary (days 6–7) phase growth in liquid culture similar to wild type L. chagasi promastigotes [10]. During the conversion from LcJ promastigotes to axenic amastigotes, an additional higher Mr MSP isoform was detected, and the relative abundance of the major MSP isoforms in amastigotes decreased, which was consistent with previous finding that amastigotes express lower levels of total MSP than promastigotes. The total abundance of MSP relative to α-tubulin was lower in amastigotes compared to stationary phase promastigotes (Figure 1C). The apparent decreased migration of MSP in the outer lanes of the promastigote and amastigote lanes are most likely due to an artifact of the gel running, since tubulin lanes followed the same pattern.
3.3. Protein profiles of promastigotes and amastigotes on two-dimensional gels
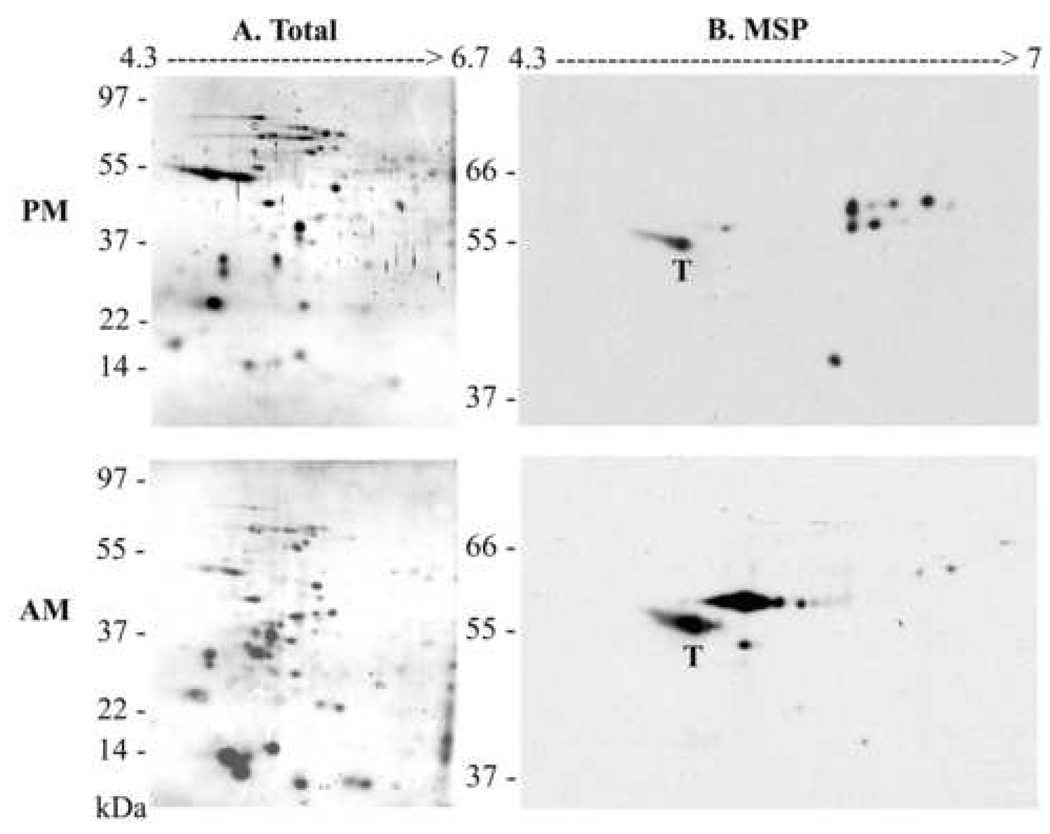
The difference between MSP proteins expressed by promastigotes versus amastigotes was further characterized on two-dimensional immunoblots (Figure 2). Parasite lysates from stationary promastigotes (20 µg) and axenic amastigotes (100 µg) were separated on the basis of pI and mass. Amastigote lysates contained a different pattern of total proteins detected by silver staining, including more prominent low Mr molecules than promastigotes (Figure 2A). MSP isoforms were identified on two-dimensional immunoblots by incubating with sheep polyclonal antiserum against L. chagasi promastigote MSP (Figure 2B). Strikingly, the predominant MSP isoforms migrated at a more acidic pI than the promastigote MSPs. This is consistent with the acidic environment of macrophage phagolysosomes in which amastigotes reside [18]. The blots were re-probed with mouse monoclonal antibody against α-tubulin, which resulted in a single spot that migrated at the same location in the blots of promastigote and amastigote lysates. The tubulin blots were overlaid on the MSP blots, and the single α-tubulin spot is labeled with a “T” in Figure 2B.
Fig. 2.
Total and MSP protein profiles of promastigotes or axenic amastigotes. A. Total lysates of stationary-phase LcJ promastigotes (PM, 20 µg) or axenic amastigotes harvested 2 weeks after stage conversion (AM, 100 µg), were separated in two dimensional electrophoresis and proteins were detected by silver staining. B. The proteins shown in panel A were transferred to nitrocellulose and probed in a western blot with sheep anti-MSP polyclonal antiserum. Shown are representative gels and immunoblots of two independent replicates conducted with different parasite protein preparations. “T” denotes the α-tubulin spot discerned on a reprobe of the first MSP blot, overlaid onto the MSP blot. The pIs and molecular mass of proteins separated on the first and second dimension are indicated at the top and the left of the gels, respectively.
3.4. Promastigote and amastigote MSP undergo different post-translational modifications
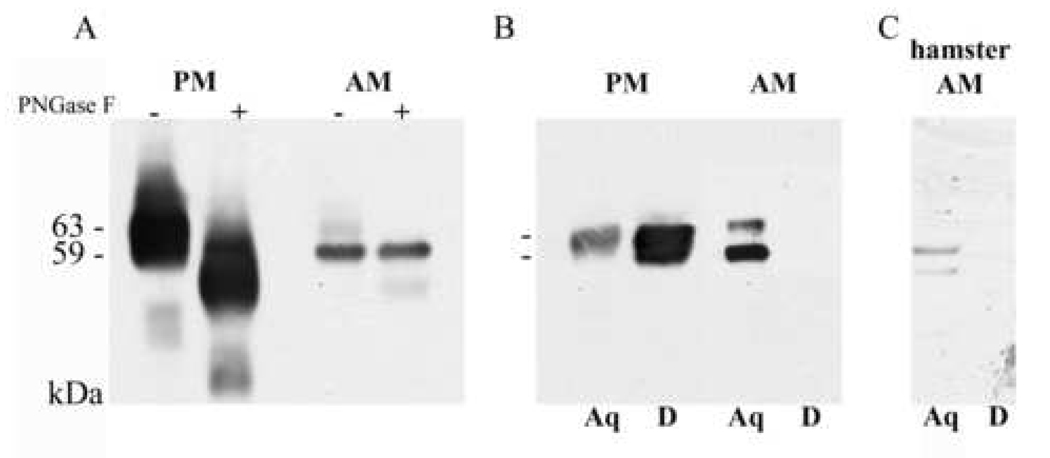
The differences in migration of the MSP isoforms on two-dimensional gels led us to ask whether these differences are due to differential MSP glycosylation or membrane anchor addition in the two parasite stages. Mature promastigote MSP proteins undergo posttranslational modifications including removal of the pro-peptide and the signal peptide, N-glycosylation, and GPI anchor addition [10, 37–39]. To determine whether amastigote MSPs are also N-glycosylated, total promastigote or amastigote lysates were treated with PNGase F, which specifically cleaves glycans from N-linked glycoproteins (Figure 3A). Consistent with the literature and our prior observations, most or all promastigote MSP isoforms were susceptible to PNGase F, documented by an increase in migration on SDS-PAGE after removal of N-linked oligosaccharides. In contrast, the major amastigote MSP isoform(s) was not affected by PNGase F treatment, although a minor isoform did increase in migration. These data suggest that, first, PNGase F is active in parasite lysates, and second, the majority of amastigote MSP is not N-glycosylated.
Fig. 3.
Post-translational modifications of LcJ promastigote or axenic amastigote MSP proteins. A. Total lysates of stationary phase promastigotes (PM) or amastigotes (AM) were untreated (−) or treated (+) with PNGase F for one hour to remove N-linked oligosaccharides, separated by SDS-PAGE, and probed on an immunoblot with sheep anti-MSP polyclonal antiserum. Increased migration indicates PNGase susceptibility. B. PM or AM cells were subjected to TX-114 fractionation and detergent insoluble material was removed by centrifugation. Proteins were separated into a detergent (D) phase enriched in amphipathic membrane molecules, or an aqueous (Aq) phase containing hydrophilic molecules. Immunoblots were incubated with polyclonal anti-MSP serum to detect MSP. C. Hamster derived amastigotes were harvested and were subjected to TX114 fractionation. Proteins were partitioned by two sequential detergent extractions into amphipathic detergent (D) or aqueous (A) phases. Proteins in each double separated phase were separated by SDS-PAGE and analyzed by western blots with anti-MSP antiserum. as indicated.
In addition to N-glycosylation, promastigote MSP is modified by addition of a GPI anchor to its C-terminal end, generating an amphipathic molecule that is soluble in detergents such as TX-114. We separated promastigote and amastigote membrane proteins with TX-114. Consistent with prior observations, promastigote MSP proteins were enriched in the detergent phase and only partially represented in the aqueous phase after detergent separation (Figure 3B). In contrast, amastigote MSP proteins were found exclusively in the aqueous phase and were undetectable in the detergent phase. The same phenomenon was observed when we used hamster-derived amastigotes, even though the signal was less due to the fewer parasites that could be obtained (Figure 3C). These data indicate that L. chagasi amastigote MSP proteins are hydrophilic in nature, suggesting they lack a typical promastigote-like GPI membrane anchor.
Amastigote proteins were solubilized in triton X-100 for the 2D-immunoblot (Figure 2), or in TX-114 prior to the deglycosylation experiment (Figure 3A–B). The higher Mr amastigote MSP seen in Figure 1C seems not to be detergent soluble (Figure 2 and Figure 3). Careful determination of molecular size showed that the predominant amastigote MSP in Figure 3A–B migrates at a similar level as the lower glycosylated promastigote MSP, as well as the higher Mr deglycosylated promastigote MSP. The same is true in Figure 2B; i.e., the non-glycosylated amastigote MSP migrates at a similar level as the lower Mr glycosylated promastigote MSP. We previously published that promastigote MSPS1 migrates at a higher Mr than MSPS2, and this difference cannot be explained by their primary sequences [26]. Although it would be possible that the major amastigote MSP in Figure 3 corresponds to a deglycosylated version of MSPS1, Figure 1B suggests MSPS gene products are not expressed in amastigotes and the data in Figure 2A suggest there could be other undetected modifications.
3.5. Subcellular fractionation of amastigotes: MSP localization
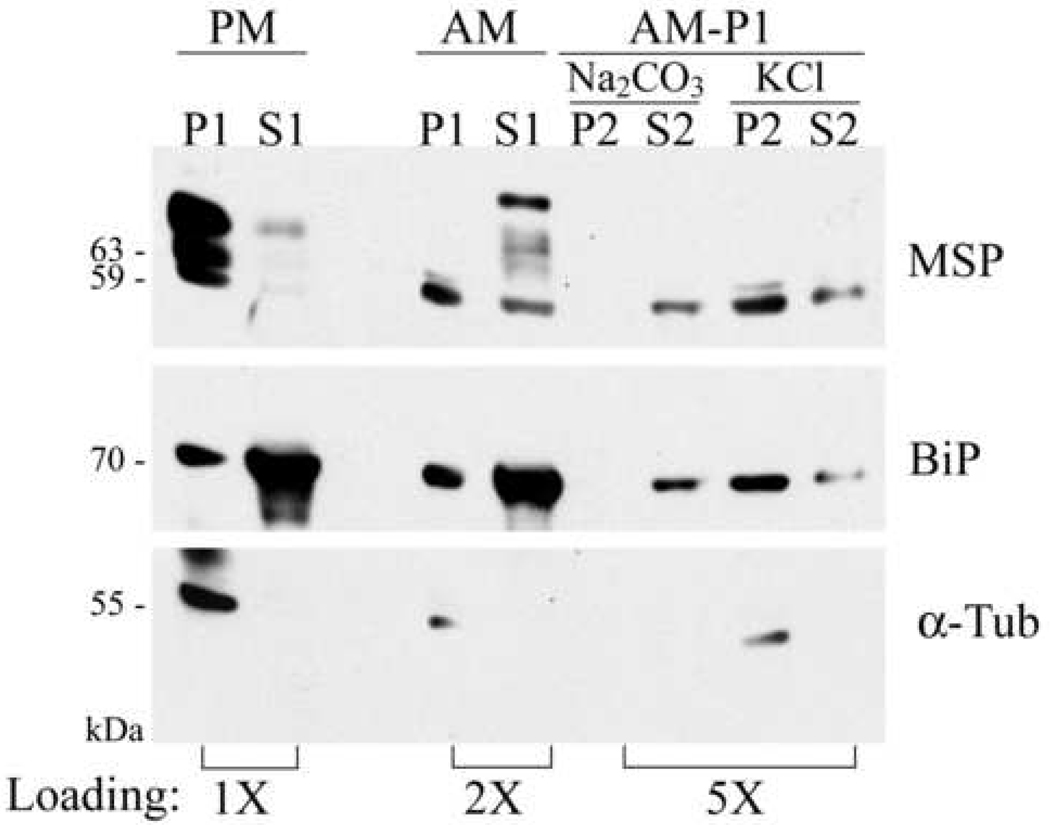
Based upon the above TX-114 results, we questioned whether amastigote MSP associates at all with parasite membranes, and if so, what is the nature of this association. Amastigotes were fractionated into cytoplasmic and membrane-containing fractions and MSP was detected on immunoblots (Figure 4). Cytosol and microsomal (membrane) fractions were normalized to contain proteins from the same number of starting parasites. As expected, the majority of promastigote MSP associated with the membrane/microsomal (P1) and not the cytoplasmic (S1) fraction. Consistent with the experiment in Figure 3, amastigote MSP localized primarily in the cytosolic fraction (S1) with some MSP of a higher Mr form than promastigote MSP, which suggested that this higher Mr amastigote MSP isoform is soluble or intracellular. A minor MSP band was associated with amastigote microsomes. This form was further characterized by alkaline and high-salt treatment. The alkaline (pH 11.5) carbonate wash procedure converts closed vesicles within microsomes into open sheets of membranes without changing the polypeptide composition of the membrane. As such, the procedure should release both peripheral membrane proteins and microsome luminal proteins [40]. In contrast, KCl only consistently releases peripheral membrane proteins [41] .
Fig. 4.
Subcellular fractionation of amastigote MSP. LcJ promastigotes (PM) or amastigotes (AM) were lysed as described in the text and separated into cytoplasmic (S1) or membrane-containing (P1) fractions. Amastigote microsomal fractions (P1) were further treated with sodium carbonate to break open organelles, or KCl to remove peripheral proteins. Carbonate or KCl insoluble materials (P2) were separated from soluble proteins (S2) by centrifugation. Proteins migrating in the various fractions were identified by immunoblot with polyclonal antisera to MSP and to BiP, or monoclonal antibody to α-tubulin (α-Tub). Cell equivalents are: 1.5 × 107 (1X) for the promastigote P1 and S1 lanes; 3 × 107 (2X) for the amastigote P1 and S1 lanes and 7.5 × 107 (5X) for the rest of the lanes.
Carbonate treatment of the amastigote microsomal fraction released all microsomal MSP into the supernatant. The endoplasmic reticulum protein BiP was also released, providing a positive control for release of intra-organelle proteins. To differentiate between organelle lumenal and peripheral membrane proteins, we performed a KCl wash to release peripheral membrane proteins. The majority of MSP remained membrane-associated after KCl treatment, suggesting MSP is not a peripheral membrane protein. The partial release of BiP parallels MSP and indicates the KCl did release some intra-organelle proteins, but the majority of BiP remained associated with the pellet (microsome). These data indicate that the majority of amastigote MSPs are soluble/cytosolic proteins, whereas a small portion of the amastigote MSP remains in the association with membranes and may be in the lumen of intracellular organelles that are broken open with sodium carbonate, pH 11.5.
3.6. Subcellular localization of promastigote and amastigote MSP
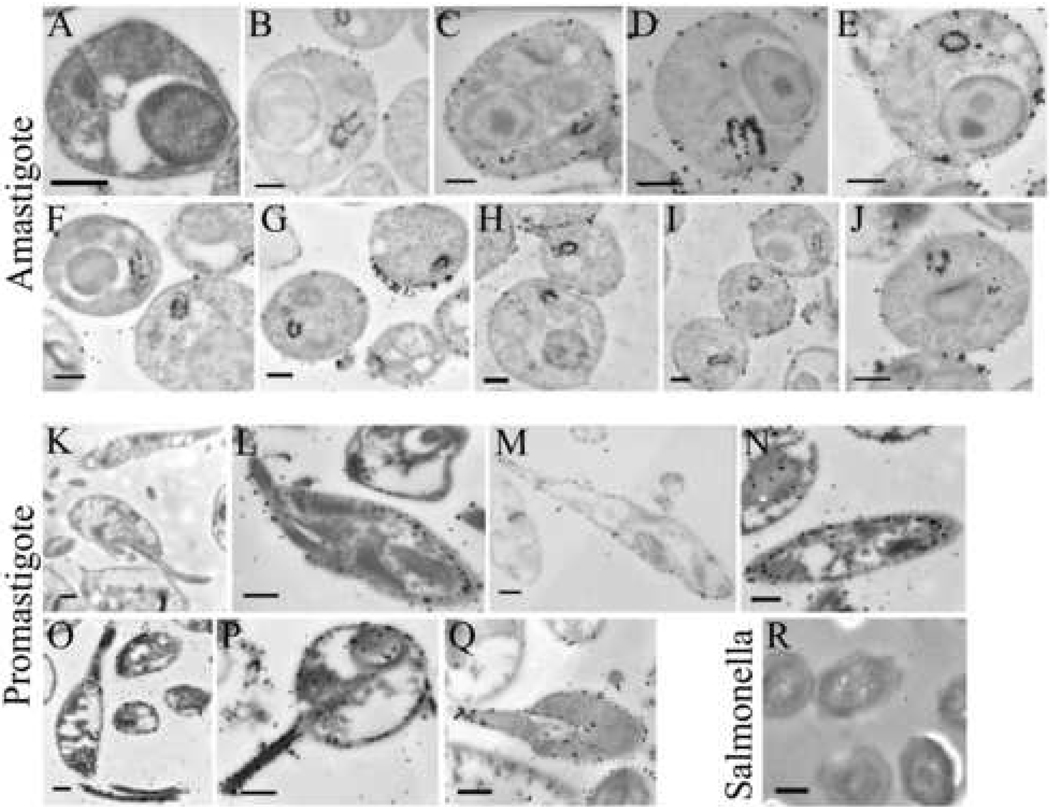
We previously reported that two-thirds of the promastigote MSP proteins are located on the surface membrane and one-third remains intracellular [9]. The above results indicated that most amastigote MSP isoforms localize intracellularly either free or in organelles. We employed immunogold electron microscopy to determine the location of MSPs in the promastigotes and amastigotes. Panels 5A and 5K are controls showing amastigotes or promastigotes, respectively, incubated with secondary antibody alone. In contrast, affinity purified sheep anti-MSP antiserum followed by secondary antibody labeled with gold particles were visualized on the surface of the LcJ promastigotes (Figure 5, L–Q), consistent with previous results with wild type L. chagasi promastigotes [9]. In contrast, much of the MSP antibody specifically labeled the flagellar pocket of amastigotes (Figure 5, B–J). This often generated a “horseshoe”-like MSP labeling pattern in the flagellar pocket of many cell sections that was not observed in promastigote cells. A lesser amount of MSP was also observed at the amastigote surface, suggesting MSP isoforms localize in both regions in axenic amastigotes. In contrast, Salmonella typhimurium was not recognized by anti-MSP antiserum (Figure 5R), indicating the specificity of the antibodies.
Fig. 5.
Localization of MSP in stationary phase LcJ promastigotes or axenic amastigotes using immunogold staining and electron microscopy. LcJ amastigotes (panels A–J) or promastigotes (panels K–Q), or Salmonella typhimurium (panel R) were fixed and embedded in LR White resins. Thin sections were incubated with buffer alone (A, K) or with affinity-purified anti-MSP antiserum (panels B–J, L–Q, R) followed by secondary antibody conjugated to ultra-small gold particles (< 0.8 nm). Scale bars represent 0.5 µm in panels A–Q, or 0.1 µm in panel R.
3.7. Extracellular release of MSP from promastigotes but not amastigotes
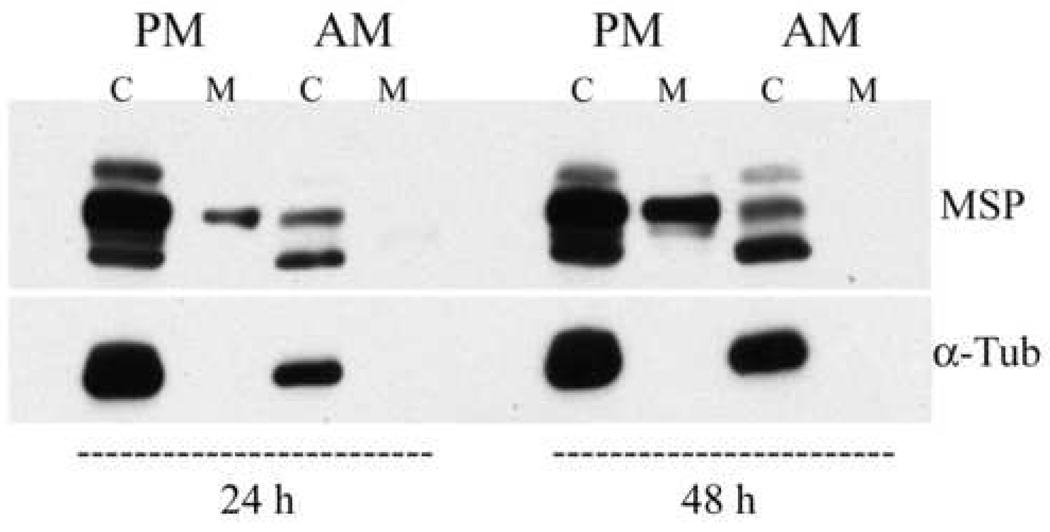
The above immunostaining results indicate that amastigote MSP proteins localize in the flagellar pocket, which is the major site of exocytosis/endocytosis in the Leishmania spp. [42, 43]. We and others have previously reported that MSP proteins are released into the extracellular medium of promastigotes [10, 44, 45], and hypothesize that extracellular MSP protease may interact with the promastigote extracellular environment. LcJ promastigotes or amastigotes were suspended in serum-free, BSA-free media for 24 or 48 h. The supernatants and cell pellets were collected, concentrated, and analyzed on immunoblots (Figure 6). Consistent with our prior observations, MSP was released into the extracellular medium of promastigotes [10]. In contrast, no release of MSP into the amastigote media was detected. The same membrane and cell pellet samples were re-probed for the presence of α-tubulin (Figure 6) and p36 (not shown). Both proteins were found in the cell pellet but not the extracellular medium, suggesting that proteins from lysed parasites did not contaminate the medium.
Fig. 6.
MSP proteins are released into the extracellular medium of promastigotes but not amastigotes. L. chagasi promastigotes (PM) or amastigotes (AM) were suspended in serum-free medium. Cells (C) and the extracellular medium (M) from the same aliquot of cells were collected after 24 and 48 hours incubation at 26°C (promastigotes) or 37°C (amastigotes). Medium was concentrated, and all samples were analyzed on immunoblots probed with sheep anti-MSP polyclonal antiserum. The same blots were re-probed with monoclonal mouse antibody against α-tubulin (α-Tub).
3.8. Metalloprotease activity in amastigotes
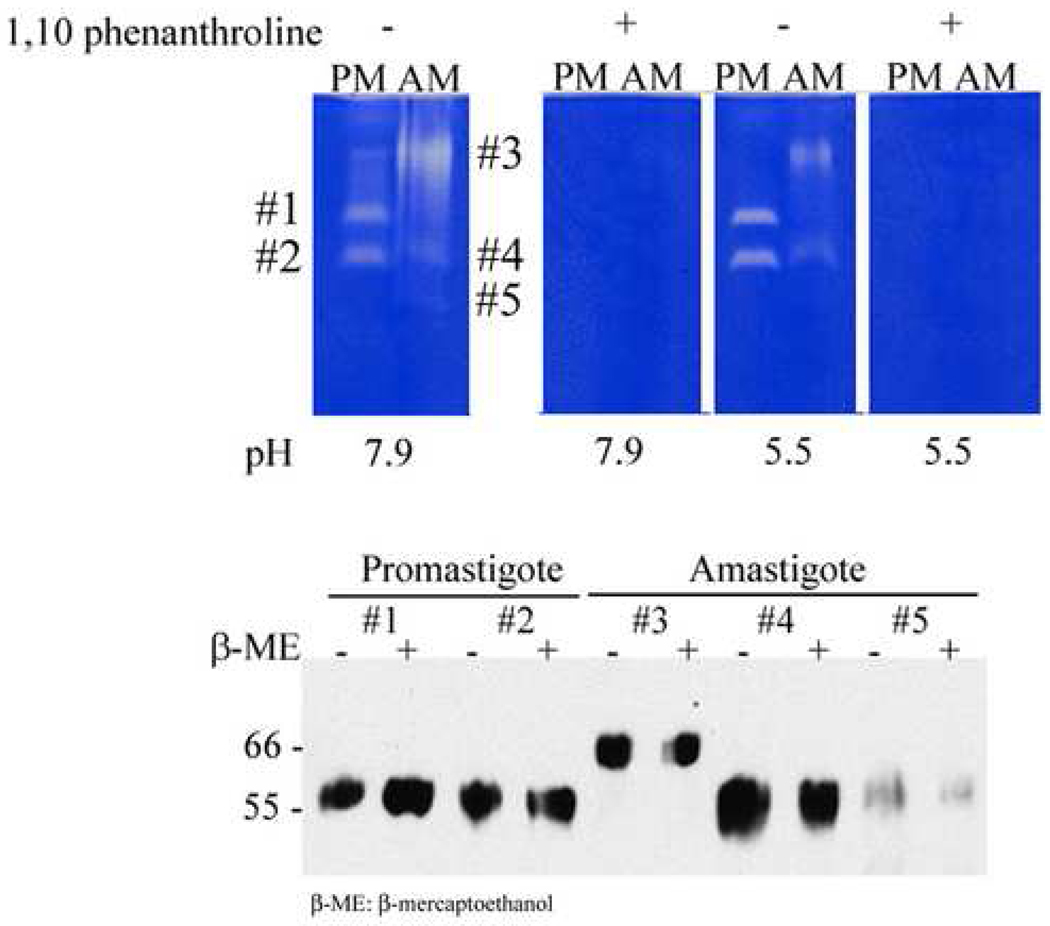
Activity of the abundant promastigote MSP metalloprotease can be detected by its gelatinolytic activity on gelatin-containing gels [8, 46]. Promastigotes are found in neutral to mild alkaline pH environments, whereas intracellular amastigotes reside in an acidified intracellular compartment [23]. Previous studies reported that the pH optimum of promastigote MSP ranges from 6 to 8 [46, 47], with a slightly lower pH optimum for MSP in amastigotes [48]. To test for metalloprotease activity, we analyzed the extracts of LcJ promastigotes and axenic amastigotes on 0.2% gelatin-containing gels at pH 7.9 and pH 5.5, incubated without or with the metalloprotease inhibitor 1,10-phenanthroline (Figure 7). Protease activity was detected in both promastigotes and amastigotes, and inhibited by 1,10-phenanthroline demonstrating the activity was due to a metalloprotease(s). Metalloprotease activity in amastigote and promastigote extracts exhibited similar activities at pH 7.9 and 5.5, verifying a widely variable pH optimum. Two major metalloprotease activity bands were apparent in the promastigote extract, the lower of which was also weakly visible in the amastigote extract. In addition, the amastigote extract contained a band with prominent protease activity migrating more slowly in the gel, and a weakly active band migrating faster than other proteases. Bands containing protease activity are labeled #1 through #5 in Figure 7.
Fig. 7.
Protease activity gels. [Top panel] Total LcJ promastigote (PM) or amastigote (AM) lysates were separated on 7.5% gels containing 0.2% gelatin. Replicate gel slices were incubated overnight in buffers at the indicated pH with or without 10 mM 1,10-phenanthroline, a metalloproteinase inhibitor. Bands corresponding to proteases were detected as clear areas in the background of gelatin staining. [Bottom panel] The major metalloprotease bands (#1 –#5) from each of the parasite forms were cut out of protease gels and analyzed on immunoblots. These were probed with sheep anti-MSP polyclonal antiserum to prove their identity.
To determine whether these promastigote and amastigote metalloproteases were truly MSPs, we cut out all predominant metalloprotease bands from a non-reducing gel and analyzed them on immunoblots probed with anti-MSP serum. All metalloprotease bands were recognized by anti-MSP antiserum. Although promastigote and the corresponding faster migrating amastigote band migrated at 60–63 kDa similar to most MSPs, surprisingly the slowly migrating amastigote metalloprotease band (band #3) corresponded to higher molecular weight MSP. The presence of β-mercaptoethanol had no effect on the migration. It is possible this band corresponds to MSPC, which is predicted to be of higher molecular size than other MSPs. When we used an antibody designed to recognize a peptide in MSPL and MSPS to probe the same blot, the high Mr MSP band was absent (data not shown), which again is consistent with the hypothesis that the predominant metalloprotease in amastigotes is MSPC.
3.9. Liquid chromatography-tandem mass spectrometric (LC-MS/MS) analysis of amastigote MSPs
L. chagasi promastigote MSP isoforms were previously identified using protein purified by separation into a TX-114 enriched fraction and adherence to concanavalin A-sepharose [10]. Isoforms were identified by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Because amastigote MSP lacks glycosylation and a GPI membrane anchor, protein purification schemes using ConA beads or TX-100 extraction were not applicable. We therefore adopted a different approach for purification. Amastigote and promastigote MSPs were immunoprecipitated with polyclonal anti-MSP serum and separated by SDS-polyacrylamide gel electrophoresis. The gel was silver stained, and bands were subjected to LC-MS/MS. The latter method combines the physical separation of liquid chromatography with mass analysis. Among all the samples sent for sequencing, one band from the promastigote samples and one from the amastigote samples were shown to contain MSP peptides. Other bands contained immunoglobulins and albumin from the immunoprecipitated preparation. MSPs in the specimens corresponded to 2–3 MSP gene products (shown in Table 1). Immunoprecipitated promastigote samples contained MSPS1, MSPC, and potentially MSPS4. Immunoprecipitated amastigote MSP contained peptides matched with MSPC. A polypeptide corresponding to MSPS4 was also recognized. Because MSPL1 and MSPS4 differ by only 2 amino acid residues, since we did not detect MSPS transcripts in amastigote RNA (Fig. 1B), and since we have not sequenced all MSPL gene products [6], it seems likely that the latter could correspond to an MSPL gene product which contains peptides corresponding to those that distinguish MSPS4. Unfortunately since a peptide corresponding to the C-terminus of MSPC was not identified on LC-MS/MS, we could not discern whether the C-terminal residues of MSPC were present or replaced with a GPI anchor.
Table 1.
Identification of promastigote and amastigote isoforms immunoprecipitated from parasite lysates using LC-MS/MS. Total amastigote or promastigote MSP was immunoprecipitated using antiserum to L. chagasi MSP, separated on 7.5% polyacrylamide gels and stained with silver stain. Bands from each parasite form were subjected to LC-MS/MS analysis at the Yale Keck Protein Facility.
| Protein analyzed |
Protein identified |
Score | Peptides Matched |
Predicted Molecular Mass** |
Mature Molecular Mass** |
|---|---|---|---|---|---|
| Amastigote #1 |
Leishmania chagasi MSPL/S4 |
198* | 7 | 63,913 | 51,754 |
| Amastigote #2 |
L. chagasi MSPC |
117* | 4 | 69,009 | 59,177 |
| Promastigote #1 |
L. chagasi MSPS1 |
338* | 8 | 63,833 | 51,674 |
| Promastigote #2 |
L. chagasi MSPS4 |
227* | 7 | 63,913 | 51,754 |
| Promastigote #3 |
L. chagasi MSPC |
111* | 3 | 69,009 | 59,177 |
Significance threshold of p< 0.05
Predicted molecular mass refers to the full length protein. Predicted mass of the mature MSP results from removal of signal peptide, pro-peptide, and (in the case of MSPL or MSPS gene products) residues C-terminal to the GPI anchor addition site [10].
4. Discussion
Many biochemical studies of the Leishmania spp. have focused on the extracellular promastigote (insect) stage, instead of the intracellular amastigote (host) stage, since promastigotes are easier to culture in vitro. To circumvent this problem, a cell-free system for amastigote propagation using L. pifanoi was first reported by Pan et al in 1984 [49]. Although it has been debated how close these are to true amastigotes, axenic amastigotes have proven useful for biochemical and molecular characterization of amastigote - host interactions. During the current study, we generated a line of L. chagasi parasites called LcJ which can convert between the two stages and replicate in vitro as axenic amastigotes. Axenic LcJ amastigotes were similar morphologically to wild type amastigotes by light microscopy, and similar biochemically in their expression of RNA encoding the amastigote-specific A2 protein, their reduced amount of the promastigote-specific transcript ARL3A RNA, and their lack of LPG [50]. LcJ amastigotes also expressed MSPC and MSPL but not MSPS transcripts, similar to our prior study of macrophage cell line-derived L. chagasi amastigotes [36]. Thus, we used this L. chagasi cell line to contrast the biochemical and biological characteristics of MSP in L. chagasi amastigotes versus promastigotes.
Several differences between amastigote and promastigote MSPs were found. The relative abundance of MSP isoforms differed on one- and two-dimensional immunoblots. In contrast to promastigote MSPs, amastigote MSP isoforms displayed a relatively acidic pI. Amastigote MSPs were non-glycosylated and lacked an amphipathic membrane anchor suggesting at least most isoforms are not linked to the parasite membrane via a GPI anchor. Most amastigote MSPs were found in a soluble non-microsomal fraction within the cells (Figure 4). The remaining amastigote MSP that was microsome-associated was contained as a soluble form within vesicles or organelles. Immuno-electron microscopy showed that amastigote MSPs are located primarily in the flagellar pocket, presumably explaining their soluble characteristics. In contrast, promastigote MSPs were predominantly surface-bound.
Amastigotes express MSPC and MSPL but not MSPS RNA according to northern blots [[36] and Figure 1]. Protease gels indicated amastigotes contain a metalloprotease that co-migrates with promastigote MSP, as well as a novel slowly migrating protease not present in promastigotes. The identity of both bands as MSP was confirmed by immunoblots of extracted protease bands. Furthermore, the larger Mr amastigote protease activity corresponded to an MSP protein of larger Mr than promastigote MSPs. LC-MS/MS data documented that amastigotes contain both MSPC and a protein that could be MSPS4, but seems more like to be an uncharacterized MSPL.
On the basis of its amino acid sequence, MSPC is predicted to migrate at a larger Mr (59,177 compared to 51,689 and 51,674 Da) and at a lower pI (5.32 versus 5.65 and 5.65) than MSPS and MSPL, respectively [10]. As such, at least one of the low pI MSPs on the amastigote 2D immunoblots could correspond to MSPC (Figure 2), as well as the high Mr MSP on immunoblots of total amastigote protein (Figure 1C), and the large proteolytically active MSP protein (Figure 7). The MSPC sequence predicts a 40-residue C-terminal extension encoding a short hydrophobic region that could be a transmembrane domain. We previously predicted the protein would be amphipathic, but the current data suggest the protein is soluble and not membrane-bound. Also surprising was the lack of N-glycosylation in amastigote MSP, since MSPC predicts four additional N-glycosylation sites compared to MSPL and MSPS. These data are consistent with the presence of a proteolytically active MSPC protein in amastigotes that could correspond to the soluble MSP protein in fractionated cells. Definitive proof of this hypothesis awaits the development of specific antisera that distinguishes MSPC from other MSP proteins.
In the Leishmania species studied, MSP is the most abundant promastigote surface protein [51]. All MSPs share high sequence identity, but there are species-specific differences in gene organization and number. L. major, a causative agent of cutaneous leishmaniasis in the Old World, has the simplest arrangement with seven MSP genes, termed gene 1 through gene 7. Genes 1 to 5 are expressed exclusively in the promastigote stage, whereas gene 6, which is most similar to L. chagasi MSPC, is expressed in both promastigotes and amastigotes and gene 7 is predominantly expressed in amastigotes and stationary phase promastigotes [52] . In L. mexicana, a cause of New World cutaneous leishmaniasis, there are ten MSP genes that fall into three classes called C1 (an MSPC homolog), C2 and C3. Promastigotes express transcripts from all the MSP classes, whereas C3 appears to be the only transcript expressed in amastigotes [53] . Other characterized MSP gene families include seven genes of L. donovani and more than 22 genes in L. guyanensis [1]. Although amastigotes often contain MSP isoforms that are different from promastigotes, there is not an exact correlation between MSP gene classes expressed in the amastigote versus promastigote stages of the different Leishmania species [42, 51–54].
Developmental changes in promastigote MSP isoforms have been documented during growth from logarithmic to stationary phase, a growth process that shares some similarities to the development of virulent metacyclic promastigotes in the sand fly gut [15, 55]. Furthermore, a form of L. mexicana MSP appeared to localize in the flagellar pocket although it is not clear which gene product this corresponds to [42]. There is evidence suggesting that MSP enhances parasite survival in macrophage phagosomes [1, 11, 18], and that intracellular MSP acts to cleave at least one macrophage cytoplasmic molecule [19]. As a result of our current studies of L. chagasi, we hypothesize that the soluble proteolytically active MSP localized in the flagellar pocket of amastigotes may play an important role in the intracellular stage of L. chagasi and could correspond to the MSPC gene product. Due to the abovementioned differences between species, we cannot predict whether there will be similar observations in other Leishmania species.
The extracellular release of MSP may be critical for promastigote pathogenicity, through such processes as cleavage of complement, extracellular matrix and antimicrobial peptides [16, 56, 57]. The absence of apparent MSP release by amastigotes was striking, and could signify an important difference in the function of this prominent protease between these parasite forms. We cannot be certain, however, whether MSP shedding occurs in vivo. As an example, secreted acid phosphatase (sAP) from Leishmania spp. promastigotes is located in the flagellar pocket [58, 59]. sAP is observed in the parasitophorous vacuole even though sAP enzymatic activity is not released in supernatants of axenically cultured parasites. It has been speculated that this enzyme may be secreted intracellularly and degrade lysosomal enzymes within macrophages [59]. We did not observe MSP proteins released into extracellular amastigote medium over a 48-hour period, but similar to sAP it is possible that such release occurs in vivo and requires additional signals such as low pH, oxidant species, or lysosomal enzymes that might be present in the macrophage.
The flagellar pocket corresponds to the major site of endocytosis and exocytosis in Leishmania spp. and other trypanosomatids. The Trypanosoma brucei flagellar pocket is responsible for distribution and internalization of the Variant Surface Glycoprotein [60]. Although most amastigote MSP was found in the flagellar pocket, some appeared to be distributed around the parasite surface (see Figure 5). Whether these MSP proteins are recycled back into amastigotes, and whether flagellar MSP serves to enzymatically cleave other host or parasite molecules, will need further investigation. Several groups have suggested that MSP may protect intracellular Leishmania spp. parasites from lysosomal hydrolases and/or provide nutrition for amastigotes in the host cell [18, 42]. The current study focused on the posttranslational modifications and localization of amastigote MSP proteins. Further studies will focus on the role of amastigote MSP isoforms in nutrient acquisition and in defense against intracellular host microbicidal functions.
Acknowledgements
The authors are grateful to Jiwen Luo and Melissa Miller for technical support of these studies. We also thank Dr. Mark Stamnes for his advice, and Dr. Bradley Jones for providing Salmonella strain for EM studies. We thank Dr. Paul Grandgenett for help with antibody purification. Microscopic studies were performed in the University of Iowa Central Microscopy Facility with the help of Dr. Kenneth Moore and Jian Shao. LC-MS/MS data were analyzed at Yale University Keck Biotechnology Resource Laboratory with the help of Dr. Kathryn Stone.
This work was supported by grants AI32135 and AI059451 from the National Institutes of Health (JED and MEW), NIH grants AI45540 and AI048822 (MEW), two Merit Review grants from the Department of Veterans’ Affairs (MEW and CY), and a Persian Gulf RFP (MEW) and an MREP (CY) from the Department of Veterans’ Affairs.
Abbreviations
- GPI
glycosylphosphatidylinositol
- MSP
major surface protease
- PAGE
polyacrylamide gel electrophoresis
- TX114
triton X-114
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yao C, Donelson JE, Wilson ME. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol Biochem Parasitol. 2003;132:1–16. doi: 10.1016/s0166-6851(03)00211-1. [DOI] [PubMed] [Google Scholar]
- 2.Bordier C. The promastigote surface protease of Leishmania. Parasitol Today. 1987;3:151–153. doi: 10.1016/0169-4758(87)90199-2. [DOI] [PubMed] [Google Scholar]
- 3.McCoy JJ, Beetham JK, Ochs DE, Donelson JE, Wilson ME. Regulatory sequences and a novel gene in the msp (GP63) gene cluster of Leishmania chagasi. Mol Biochem Parasitol. 1998;95:251–265. doi: 10.1016/s0166-6851(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 4.Roberts SC, Swihart KG, Agey MW, et al. Sequence diversity and organization of the msp gene family encoding gp63 of Leishmania chagasi. Mol Biochem Parasitol. 1993;62:157–171. doi: 10.1016/0166-6851(93)90106-8. [DOI] [PubMed] [Google Scholar]
- 5.Wilson ME, Hardin KK, Donelson JE. Expression of the major surface glycoprotein of Leishmania donovani chagasi in virulent and attenuated promastigotes. J Immunol. 1989;143:678–684. [PubMed] [Google Scholar]
- 6.Ramamoorthy R, Donelson JE, Paetz KE, et al. Three distinct RNAs for the surface protease gp63 are differentially expressed during development of Leishmania donovani chagasi promastigotes to an infectious form. J Biol Chem. 1992;267:1888–1895. [PubMed] [Google Scholar]
- 7.Pearson RD, Wheeler DA, Harrison LH, Kay HD. The immunobiology of leishmaniasis. Rev Infect Dis. 1983;5:907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- 8.Bouvier J, Etges RJ, Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem. 1985;260:15504–15509. [PubMed] [Google Scholar]
- 9.Yao C, Luo J, Hsiao C, Donelson JE, Wilson ME. Internal and surface subpopulations of the major surface protease (MSP) of Leishmania chagasi. Mol Biochem Parasitol. 2005;139:173–183. doi: 10.1016/j.molbiopara.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Yao C, Luo J, Storlie P, Donelson JE, Wilson ME. Multiple products of the Leishmania chagasi major surface protease (MSP or GP63) gene family. Mol Biochem Parasitol. 2004;135:171–183. doi: 10.1016/j.molbiopara.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Seay MB, Heard PL, Chaudhuri G. Surface Zn-proteinase as a molecule for defense of Leishmania mexicana amazonensis promastigotes against cytolysis inside macrophage phagolysosomes. Infect Immun. 1996;64:5129–5137. doi: 10.1128/iai.64.12.5129-5137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimenta PF, Saraiva EM, Sacks DL. The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major. Exp Parasitol. 1991;72:191–204. doi: 10.1016/0014-4894(91)90137-l. [DOI] [PubMed] [Google Scholar]
- 13.Russell DG. The macrophage-attachment glycoprotein gp63 is the predominant C3-acceptor site on Leishmania mexicana promastigotes. Eur J Biochem. 1987;164:213–221. doi: 10.1111/j.1432-1033.1987.tb11013.x. [DOI] [PubMed] [Google Scholar]
- 14.Russell DG, Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986;136:2613–2620. [PubMed] [Google Scholar]
- 15.Yao C, Leidal KG, Brittingham A, et al. Biosynthesis of the major surface protease GP63 of Leishmania chagasi. Mol Biochem Parasitol. 2002;121:119–128. doi: 10.1016/s0166-6851(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 16.Brittingham A, Morrison CJ, McMaster WR, et al. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;155:3102–3111. [PubMed] [Google Scholar]
- 17.Chaudhuri G, Chang KP. Acid protease activity of a major surface membrane glycoprotein (gp63) from Leishmania mexicana promastigotes. Mol Biochem Parasitol. 1988;27:43–52. doi: 10.1016/0166-6851(88)90023-0. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri G, Chaudhuri M, Pan A, Chang KP. Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J Biol Chem. 1989;264:7483–7489. [PubMed] [Google Scholar]
- 19.Corradin S, Ransijn A, Corradin G, et al. MARCKS-related protein (MRP) is a substrate for the Leishmania major surface protease leishmanolysin (gp63) J Biol Chem. 1999;274:25411–25418. doi: 10.1074/jbc.274.36.25411. [DOI] [PubMed] [Google Scholar]
- 20.Wilson ME, Hardin KK. The major concanavalin A-binding surface glycoprotein of Leishmania donovani chagasi promastigotes is involved in attachment to human macrophages. J Immunol. 1988;141:265–272. [PubMed] [Google Scholar]
- 21.Balanco JM, Pral EM, da Silva S, et al. Axenic cultivation and partial characterization of Leishmania braziliensis amastigote-like stages. Parasitology. 1998;116(Pt 2):103–113. doi: 10.1017/s003118209700214x. [DOI] [PubMed] [Google Scholar]
- 22.Hodgkinson VH, Soong L, Duboise SM, McMahon-Pratt D. Leishmania amazonensis: cultivation and characterization of axenic amastigote-like organisms. Exp Parasitol. 1996;83:94–105. doi: 10.1006/expr.1996.0053. [DOI] [PubMed] [Google Scholar]
- 23.Debrabant A, Joshi MB, Pimenta PF, Dwyer DM. Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int J Parasitol. 2004;34:205–217. doi: 10.1016/j.ijpara.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Somanna A, Mundodi V, Gedamu L. In vitro cultivation and characterization of Leishmania chagasi amastigote-like forms. Acta Trop. 2002;83:37–42. doi: 10.1016/s0001-706x(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 25.Goyard S, Segawa H, Gordon J, et al. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol. 2003;130:31–42. doi: 10.1016/s0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 26.Roberts SC, Wilson ME, Donelson JE. Developmentally regulated expression of a novel 59-kDa product of the major surface protease (Msp or gp63) gene family of Leishmania chagasi. J Biol Chem. 1995;270:8884–8892. doi: 10.1074/jbc.270.15.8884. [DOI] [PubMed] [Google Scholar]
- 27.Zarley JH, Britigan BE, Wilson ME. Hydrogen peroxide-mediated toxicity for Leishmania donovani chagasi promastigotes. Role of hydroxyl radical and protection by heat shock. J Clin Invest. 1991;88:1511–1521. doi: 10.1172/JCI115461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boddicker JD, Jones BD. Lon protease activity causes down-regulation of Salmonella pathogenicity island 1 invasion gene expression after infection of epithelial cells. Infect Immun. 2004;72:2002–2013. doi: 10.1128/IAI.72.4.2002-2013.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill KL, Hutchings NR, Grandgenett PM, Donelson JE. T lymphocyte-triggering factor of african trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J Biol Chem. 2000;275:39369–39378. doi: 10.1074/jbc.M006907200. [DOI] [PubMed] [Google Scholar]
- 30.Tolson DL, Turco SJ, Pearson TW. Expression of a repeating phosphorylated disaccharide lipophosphoglycan epitope on the surface of macrophages infected with Leishmania donovani. Infect Immun. 1990;58:3500–3507. doi: 10.1128/iai.58.11.3500-3507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 33.Ip HS, Orn A, Russell DG, Cross GA. Leishmania mexicana mexicana gp63 is a site-specific neutral endopeptidase. Mol Biochem Parasitol. 1990;40:163–172. doi: 10.1016/0166-6851(90)90038-n. [DOI] [PubMed] [Google Scholar]
- 34.Charest H, Zhang WW, Matlashewski G. The developmental expression of Leishmania donovani A2 amastigote-specific genes is post-transcriptionally mediated and involves elements located in the 3'-untranslated region. J Biol Chem. 1996;271:17081–17090. doi: 10.1074/jbc.271.29.17081. [DOI] [PubMed] [Google Scholar]
- 35.Cuvillier A, Redon F, Antoine JC, et al. LdARL-3A, a Leishmania promastigote-specific ADP-ribosylation factor-like protein, is essential for flagellum integrity. J Cell Sci. 2000;113(Pt 11):2065–2074. doi: 10.1242/jcs.113.11.2065. [DOI] [PubMed] [Google Scholar]
- 36.Streit JA, Donelson JE, Agey MW, Wilson ME. Developmental changes in the expression of Leishmania chagasi gp63 and heat shock protein in a human macrophage cell line. Infect Immun. 1996;64:1810–1818. doi: 10.1128/iai.64.5.1810-1818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilley JD, Zawadzki JL, McConville MJ, Coombs GH, Mottram JC. Leishmania mexicana mutants lacking glycosylphosphatidylinositol (GPI):protein transamidase provide insights into the biosynthesis and functions of GPI-anchored proteins. Mol Biol Cell. 2000;11:1183–1195. doi: 10.1091/mbc.11.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macdonald MH, Morrison CJ, McMaster WR. Analysis of the active site and activation mechanism of the Leishmania surface metalloproteinase GP63. Biochim Biophys Acta. 1995;1253:199–207. doi: 10.1016/0167-4838(95)00155-5. [DOI] [PubMed] [Google Scholar]
- 39.Olafson RW, Thomas JR, Ferguson MA, et al. Structures of the N-linked oligosaccharides of Gp63, the major surface glycoprotein, from Leishmania mexicana amazonensis. J Biol Chem. 1990;265:12240–12247. [PubMed] [Google Scholar]
- 40.Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci. 1993;105(Pt 4):1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- 42.Medina-Acosta E, Karess RE, Schwartz H, Russell DG. The promastigote surface protease (gp63) of Leishmania is expressed but differentially processed and localized in the amastigote stage. Mol Biochem Parasitol. 1989;37:263–273. doi: 10.1016/0166-6851(89)90158-8. [DOI] [PubMed] [Google Scholar]
- 43.Overath P, Stierhof YD, Wiese M. Endocytosis and secretion in trypanosomatid parasites - tumultuous traffic in a pocket. Trends in Cell Biology. 1997;7:27–33. doi: 10.1016/S0962-8924(97)10046-0. [DOI] [PubMed] [Google Scholar]
- 44.Jaffe CL, Dwyer DM. Extracellular release of the surface metalloprotease, gp63, from Leishmania and insect trypanosomatids. Parasitol Res. 2003;91:229–237. doi: 10.1007/s00436-003-0960-0. [DOI] [PubMed] [Google Scholar]
- 45.McGwire BS, O'Connell WA, Chang KP, Engman DM. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: implications for parasite virulence. J Biol Chem. 2002;277:8802–8809. doi: 10.1074/jbc.M109072200. [DOI] [PubMed] [Google Scholar]
- 46.Cuevas IC, Cazzulo JJ, Sanchez DO. gp63 homologues in Trypanosoma cruzi: surface antigens with metalloprotease activity and a possible role in host cell infection. Infect Immun. 2003;71:5739–5749. doi: 10.1128/IAI.71.10.5739-5749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etges R, Bouvier J, Bordier C. The major surface protein of Leishmania promastigotes is a protease. J Biol Chem. 1986;261:9098–9101. [PubMed] [Google Scholar]
- 48.Glew RH, Saha AK, Das S, Remaley AT. Biochemistry of the Leishmania species. Microbiol Rev. 1988;52:412–432. doi: 10.1128/mr.52.4.412-432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan AA. Leishmania mexicana: serial cultivation of intracellular stages in a cell-free medium. Exp Parasitol. 1984;58:72–80. doi: 10.1016/0014-4894(84)90022-5. [DOI] [PubMed] [Google Scholar]
- 50.Zhang WW, Charest H, Ghedin E, Matlashewski G. Identification and overexpression of the A2 amastigote-specific protein in Leishmania donovani. Mol Biochem Parasitol. 1996;78:79–90. doi: 10.1016/s0166-6851(96)02612-6. [DOI] [PubMed] [Google Scholar]
- 51.Bahr V, Stierhof YD, Ilg T, et al. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol. 1993;58:107–121. doi: 10.1016/0166-6851(93)90095-f. [DOI] [PubMed] [Google Scholar]
- 52.Voth BR, Kelly BL, Joshi PB, Ivens AC, McMaster WR. Differentially expressed Leishmania major gp63 genes encode cell surface leishmanolysin with distinct signals for glycosylphosphatidylinositol attachment. Mol Biochem Parasitol. 1998;93:31–41. doi: 10.1016/s0166-6851(98)00013-9. [DOI] [PubMed] [Google Scholar]
- 53.Medina-Acosta E, Karess RE, Russell DG. Structurally distinct genes for the surface protease of Leishmania mexicana are developmentally regulated. Mol Biochem Parasitol. 1993;57:31–45. doi: 10.1016/0166-6851(93)90241-o. [DOI] [PubMed] [Google Scholar]
- 54.Ilg T, Harbecke D, Overath P. The lysosomal gp63-related protein in Leishmania mexicana amastigotes is a soluble metalloproteinase with an acidic pH optimum. FEBS Lett. 1993;327:103–107. doi: 10.1016/0014-5793(93)81049-6. [DOI] [PubMed] [Google Scholar]
- 55.Davies CR, Cooper AM, Peacock C, Lane RP, Blackwell JM. Expression of LPG and GP63 by different developmental stages of Leishmania major in the sandfly Phlebotomus papatasi. Parasitology. 1990;101(Pt 3):337–343. doi: 10.1017/s0031182000060522. [DOI] [PubMed] [Google Scholar]
- 56.Kulkarni MM, McMaster WR, Kamysz E, et al. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol Microbiol. 2006;62:1484–1497. doi: 10.1111/j.1365-2958.2006.05459.x. [DOI] [PubMed] [Google Scholar]
- 57.McGwire BS, Chang KP, Engman DM. Migration through the extracellular matrix by the parasitic protozoan Leishmania is enhanced by surface metalloprotease gp63. Infect Immun. 2003;71:1008–1010. doi: 10.1128/IAI.71.2.1008-1010.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukhopadhyay S, Mandal C. Glycobiology of Leishmania donovani. Indian J Med Res. 2006;123:203–220. [PubMed] [Google Scholar]
- 59.Stierhof YD, Schwarz H, Menz B, et al. Monoclonal antibodies to Leishmania mexicana promastigote antigens. II. Cellular localization of antigens in promastigotes and infected macrophages. J Cell Sci. 1991;99(Pt 1):181–186. doi: 10.1242/jcs.99.1.181. [DOI] [PubMed] [Google Scholar]
- 60.Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol. 2004;53:735–744. doi: 10.1111/j.1365-2958.2004.04224.x. [DOI] [PubMed] [Google Scholar]