Abstract
The potential functional roles of M3 muscarinic receptors in mouse atria were examined by pharmacological and molecular biological techniques, using wild-type mice, muscarinic M2 or M3 receptor single knockout (M2KO, M3KO), and M2 and M3 muscarinic receptor double knockout mice (M2/M3KO). Real-time quantitative reverse transcriptase-polymerase chain reaction analysis showed that the M2 receptor mRNA was expressed predominantly in mouse atria but that the M1, M3, M4, and M5 receptor subtypes were also expressed at low levels. Carbachol (10 nM–30 μM) decreased the spontaneous beating frequency of right atria isolated from wild-type mice. Studies with subtype-preferring antagonists and atria from M2KO mice confirmed that this activity is mediated by the M2 receptor subtype. In left atria from wild-type mice, carbachol decreased the amplitude of electrical field stimulation-evoked contractions (negative inotropic action), but this inhibition was transient and was followed by a gradual increase in contraction amplitude (positive inotropic response). In atria from M3KO mice, the transient negative inotropic action of carbachol changed to a sustained negative inotropic action. In contrast, in atria from M2KO mice, carbachol showed only positive inotropic activity. In atria from M2/M3 double KO mice, carbachol was devoid of any inotropic activity. These observations, complemented by functional studies with subtype-preferring antagonists, convincingly demonstrate that atrial M3 muscarinic receptors mediate positive inotropic effects in mouse atria. Physiologically, this activity may serve to dampen the inhibitory effects of M2 receptor activation on atrial contractility.
Muscarinic receptor stimulation by acetylcholine plays an important role in parasympathetic control of cardiac functions such as heart rate (chronotropic action), conduction velocity (dromotropic action), and contractility (inotropic action). Muscarinic receptors are prototypic members of the superfamily of G protein-coupled receptors, and molecular cloning studies have demonstrated the existence of five distinct mammalian muscarinic receptor subtypes (M1–M5) (Caulfield and Birdsall, 1998). Based on their differential G protein-coupling properties, the five receptors can be subdivided into two major functional classes. The M1, M3, and M5 receptors preferentially couple to Gq/11 proteins, whereas the M2 and M4 receptors are selectively linked to Gi/o proteins (Caulfield and Birdsall, 1998; Lanzafame et al., 2003). It is well documented that the heart predominantly expresses the M2 receptor subtype (Brodde and Michel, 1999; Dhein et al., 2001). After activation of cardiac M2 receptors, the activated α subunit of Gi proteins inhibits adenylate cyclase activity, resulting in a decrease of cytoplasmic cAMP, whereas the βγ subunit of the Gi proteins directly activates the inwardly rectifying muscarinic K+ channel (Yamada et al., 1998; Dhein et al., 2001). However, the M2 receptor is not the only muscarinic receptor subtype present in the heart (Wang et al., 2004). Pharmacological, biochemical, immunohistochemical, and molecular biological studies using whole heart tissues or isolated cardiomyocytes indicate that the heart, especially cardiomyocytes, also expresses low levels of non-M2 muscarinic receptors, including the M1 and M3 receptor subtypes (Gallo et al., 1993; Sharma et al., 1996; Hardouin et al., 1998; Hellgren et al., 2000; Wang et al., 2001, 2004; Krejcí and Tucek, 2002; Pönicke et al., 2003; Willmy-Matthes et al., 2003; Pérez et al., 2006; Myslivecek et al., 2008).
At present, little is known about the functional roles of the non-M2 muscarinic receptors that are present in the heart. It has been suggested that the M1 receptor causes positive inotropic effects by increasing the activity of the L-type Ca2+ currents in guinea pig myocytes (Gallo et al., 1993) or by increasing Ca2+ release in rat myocytes (Sharma et al., 1996). It has also been proposed that M1 or M3 receptors mediate positive inotropic responses in isolated cardiac muscle strips from human and mouse (Du et al., 1995; Nishimaru et al., 2000). However, studies in this area have been hampered by the lack of small-molecule ligands that can block or activate specific muscarinic receptor subtypes with high selectivity.
Recently, mutant mice lacking M1 to M5 muscarinic receptors (knockout mice) have become available as novel experimental tools to study the functional roles of individual muscarinic receptors in the heart and other tissues and organs (Wess, 2004). In spontaneously beating right atria from M2 receptor knockout (M2KO) mice, carbachol was devoid of any negative chronotropic activity (Stengel et al., 2000), in agreement with previous evidence indicating that the M2 receptor mediates the negative chronotropic actions of muscarinic agonists (Caulfield and Birdsall, 1998). In electrically stimulated atria from wild-type mice, acetylcholine was shown to induce both positive and negative inotropic responses (Nishimaru et al., 2000; Tanaka et al., 2001). Pertussis toxin treatment selectively abolished the negative inotropic activity of acetylcholine (Nishimaru et al., 2000), suggesting that one or more of the odd-numbered muscarinic receptors (M1, M3, and M5) that couple to pertussis toxin-insensitive, Gq/11-type proteins mediate the positive inotropic action of acetylcholine.
In the present study, we used muscarinic M2 or M3 receptor single knockout (M2KO and M3KO) and M2 and M3 muscarinic receptor double knockout mice (M2/M3KO) to study the potential role of the M3 receptor subtype in modulating atrial contractility. These experiments were complemented by functional studies using subtype-preferring antagonists and real-time qRT-PCR expression studies. Our data clearly demonstrate that atrial M3 muscarinic receptors mediate the positive inotropic effects of muscarinic agonists in mouse atria. This finding further highlights the usefulness of muscarinic receptor knockout mice to study different aspects of cardiac function.
Materials and Methods
Animals and Tissue Preparations. All experiments were performed in accordance with the institutional guidelines approved by the Animal Ethics Committee of the School of Veterinary Medicine (Rakuno Gakuen University, Ebetsu, Hokkaido, Japan).
Wild-type mice (DDY strain; Sankyo Lab Service, Sapporo, Japan) as well as mice lacking either the M2 (M2KO) or the M3 receptor (M3KO) or both M2 and M3 receptors (M2/M3KO), and the corresponding wild-type mice were used in the present experiments (either male or female). The generation of M2KO, M3KO, and M2/M3KO mice has been described previously (Gomeza et al., 1999; Yamada et al., 2001; Struckmann et al., 2003). The genetic backgrounds of the mice used in the present study were 129J1 (50%) × CF1 (50%) for M2KO and their corresponding wild-type mice, 129vEv (50%) × CF1 (50%) for M3KO and their corresponding wild-type mice, and 129J1(25%) × 129SvEv (25%) × CF1 (50%) for M2/M3KO mice. The animals were housed in ventilated polycarbonate cages. The temperature of the animal room was maintained at 23 ± 1°C, with a relative humidity of 40 to 60% and a daily light/dark cycle (7:00 AM–7:00 PM). Food (CRF-1; Oriental Yeast Co. Ltd., Tokyo, Japan) and water were given ad libitum.
Adult mice that were more than 3 months old (weight, 23–30 g) were killed by cervical dislocation. The beating heart was isolated from each animal and immersed in warmed bubbling Krebs' solution. Spontaneously beating left and right atria were dissected together from ventricles and their lumens were rinsed well to remove blood. The left atrium was attached with thread to a stationary glass rod, whereas the right atrium was tied with thread to a force displacement transducer. The atria were placed in an organ bath containing 20 ml of Krebs' solution (118 mM NaCl, 4.75 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 25 mM NaHCO2, and 11.5 mM glucose) warmed at 37°C and gassed with 95% O2 + 5% CO2. After a 60-min equilibration period, the effect of carbachol on spontaneous beating frequency was investigated to analyze chronotropic activity. To study inotropic responses, the left atrium was isolated rapidly and washed well using a fresh bubbling Krebs' solution. The atrium was placed between a pair of platinum rod electrodes and suspended vertically in an organ bath. The end of the preparation was tied and connected to a force displacement transducer. Electrical field stimulation (EFS; 1 Hz, 2 ms in duration, 1.5× threshold voltage; Nishimaru et al., 2000; Tanaka et al., 2001) was applied by an electrical stimulator. After a steady EFS-induced contraction was established, carbachol was applied cumulatively at 3-min intervals or noncumulatively (single applications at 30-min intervals), and carbachol concentration-response curves for inotropic responses were constructed. The concentration-response curves were analyzed by sigmoidal nonlinear regression fit using Origin 7.0 (OriginLab Corp., Northampton, MA) to determine the carbachol concentrations producing 50% (EC50) of its maximal effect (Emax). Because there were no significant differences in heart rate and responsiveness to carbachol among the different wild-type mice examined (M2WT, M3WT, and M2/M3WT), data obtained with these mice were generally pooled.
We also studied the effects of several muscarinic receptor antagonists on carbachol-induced atrial responses. After obtaining control responses to carbachol, preparations were pretreated with the antagonist, and then carbachol concentration-response curves were constructed in the presence of the antagonist. Antagonist dissociation constants (pKb) were determined by using the following equation: pKb = log(CR - 1) - log[antagonist], where [antagonist] denotes the molar concentration of the antagonist, and CR is the ratio of the EC50 value of carbachol in the presence of the antagonist divided by that in the absence of the antagonist.
To examine the involvement of pertussis toxin-sensitive G proteins in the carbachol-induced negative chronotropic and negative inotropic actions, DDY mice were injected with pertussis toxin (300 μg/kg i.p.) 96 h before experiments. Treatment with pertussis toxin did not cause any obvious behavioral changes or toxic effects. After 96 h, atria were isolated, and the inotropic and chronotropic responses to carbachol were investigated and compared with those of control atria from nonpertussis toxin-treated DDY mice.
Analysis of the mRNA Expression of Muscarinic Receptor Subtypes. Isolated cardiac ventricles and atria from DDY mice were cut into small pieces and were immediately immersed in RNAlater (RNA stabilization solution; Takara, Kyoto, Japan) for 12 h at 4°C and then stored at -30°C until use. Total RNA was extracted by the acid-guanidine-phenol-chloroform method (TRIzol reagent; Invitrogen, Carlsbad, CA). For quantitative analysis of muscarinic receptor mRNA expression levels, real-time PCR was conducted using SYBR Green. All primer pairs used were custom-synthesized, and their sequences were 5′-TCAGGACTCCTCTGGCTTC-3′ (forward) and 5′-CCGGGTTTCACTCTCTGTCT-3′ (reverse) for the M1 receptor (GenBank NM_007698); 5′-CCGGTGTCTCCCAGTCTAGT-3′ (forward) and 5′-CAGACGTGGAGTCATTGGAG-3′ (reverse) for the M2 receptor (GenBank NM_203491); 5′-ACCAAGCTACCCTCCTCAGA-3′ (forward) and 5′-GACAGTTGTCACGGTCATCC-3′ (reverse) for the M3 receptor (GenBank NM_033269); 5′-ATGGTGTTCATTGCGACAGT-3′ (forward) and 5′-GACTGTCTGCAACTGCCTGT-3′ (reverse) for the M4 receptor (GenBank NM_007699); and 5′-CGATCATGATGCCAGCCCTCT-3′ (forward) and 5′-GACTGTCTGCAACTGCCTGT-3′ (reverse) for the M5 receptor (GenBank NM_205783). PCR amplification was carried out in a total volume of 20 μl containing Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). The assay was performed using an Opticon Chromo 4 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA), and the PCR cycling program consisted of 2 min at 50°C, 2 min at 95°C, 50 cycles of 15 s at 95°C, and 1 min at 61.4°C. Plate reading was carried out at 61.4°C. Melting curve analysis after PCR amplification confirmed the specificity of the primers by detection of a single PCR product of the expected size on 2% agarose gels. PCR reactions were carried out in duplicate in 96-well plates. Standard curves were obtained using PCR fragments that had been isolated using a PCR purification kit, resuspended in 10 mM Tris-EDTA buffer, pH 8.0, and quantified with a spectrophotometer to calculate cDNA concentration. Standard curves of five points consisted of 10-fold serial dilutions. Sample concentrations calculated from the standard curves were converted into copies of receptor cDNA per 1 μg of RNA.
Chemicals. The following chemicals were used: atropine sulfate (Wako Pure Chemicals, Osaka, Japan), carbamylcholine chloride (carbachol; Sigma-Aldrich, St. Louis, MO), AF-DX116 (Tocris Bioscience, Ellisville, MO), 4-diphenylacetoxy-N-methyl-piperidine (4-DAMP; Tocris Bioscience), p-fluorohexahydrosiladiphenidol hydrochloride (p-F-HHSiD; Tocris Bioscience), indomethacin (Sigma-Aldrich), and pertussis toxin (Wako Pure Chemicals). AF-DX116 and indomethacin were dissolved in dimethyl sulfoxide and diluted with distilled water. The maximum concentration of dimethyl sulfoxide in the bathing solution was kept below 0.05%, a concentration at which vehicle did not change spontaneous atrial beating frequency and EFS-induced contractions. Pertussis toxin was dissolved in sterilized saline and was given to mice (300 μg/kg i.p.) in a volume of 0.2 ml.
Statistics. Data are expressed as means ± S.E.M. of at least four independent experiments using left and right atria isolated from different mice. The significance of differences between two or more groups was determined by Student's t test (paired and unpaired) or one-way analysis of variance using post hoc Bonferroni's test. The significance of differences was determined at p < 0.05.
Results
Expression of Muscarinic Receptors in the Mouse Heart. We carried out real-time qRT-PCR studies to examine the expression of M1 to M5 muscarinic receptor mRNAs in the mouse heart. As shown in Table 1, M2 receptor mRNA expression was predominant both in the ventricle and atrium, but M1, M3, M4, and M5 receptor mRNAs were also expressed at low levels. Total receptor mRNA expression levels were similar in atria and ventricles.
TABLE 1.
Expression of muscarinic receptor mRNAs in the mouse atrium (left and right) or ventricle studied by real-time qRT-PCR Values are means ± S.E.M. of four tissues from different animals. Total copies of muscarinic receptor transcripts in the atrium were not significantly different from those in the ventricle. For details, see Materials and Methods.
| Receptor Subtype | Copies/Microgram RNA | % Expression | |
|---|---|---|---|
| Atrium | M1 | 1044 ± 290 | 0.03 |
| M2 | 3,436,720 ± 198,800 | 99.87 | |
| M3 | 1622 ± 1009 | 0.05 | |
| M4 | 1038 ± 133 | 0.03 | |
| M5 | 902 ± 154 | 0.02 | |
| Ventricle | M1 | 2433 ± 478 | 0.06 |
| M2 | 3,416,230 ± 883,540 | 99.8 | |
| M3 | 2470 ± 542 | 0.06 | |
| M4 | 1994 ± 858 | 0.05 | |
| M5 | 1288 ± 505 | 0.03 |
Effects of Carbachol on Spontaneously Beating Atria from Wild-Type (DDY) Mice. In spontaneously beating right atria of DDY mice (380 ± 25 beats/min; n = 7), cumulative administration of carbachol (10 nM–10 μM) caused concentration-dependent inhibition of spontaneous beating frequency and finally abolished atrial beating activity (EC50 = 429 ± 96 nM; n = 7) (Fig. 1) .The inhibition of spontaneous beating by carbachol lasted for 15 to 20 min without recovery. AF-DX116 (1 μM) shifted the concentration-response curves of carbachol to the right (EC50 = 13,570 ± 3640 nM; n = 5) without change in Emax values (calculated pKb, 7.54 ± 0.1; n = 5) (Fig. 1). We also examined negative chronotropic responses to carbachol in atria isolated from pertussis toxin-treated DDY mice. Pertussis toxin treatment had no significant effect on spontaneous beating frequency (364 ± 24 beats/min; n = 8) but abolished the negative chronotropic responses to carbachol. The spontaneous beating frequency tended to increase at high concentrations of carbachol (10–100 μM), but this effect was not statistically significant, compared with the control values (Fig. 1).
Fig. 1.
Chronotropic responses to carbachol in spontaneously beating right atria of DDY mice. Concentration-chronotropic response relationships of carbachol were constructed in right atria isolated from nontreated (control; •) and pertussis toxin-treated (PTX; ▴) wild-type mice. AF-DX116 (1 μM) competitively antagonized the negative chronotropic response to carbachol (○). Pertussis toxin treatment did not change spontaneous beating frequency (control, 380 ± 25, n = 7; pertussis toxin, 364 ± 24, n = 8). Ordinate, heart rate expressed as percentage of baseline. Abscissa, carbachol concentration (log M). Symbols and vertical bars are means and S.E.M. of five to eight experiments.
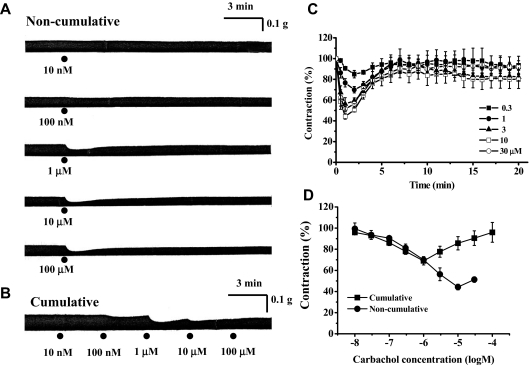
Effects of Carbachol on EFS-Induced Contractions of Atria from Wild-Type (DDY) Mice. Left atria from wild-type (DDY) mice were stimulated electrically via EFS, and the effect of carbachol on the magnitude of EFS-evoked contractions was examined. Carbachol (10 nM–100 μM) inhibited EFS-induced atrial contractions in a concentration-dependent manner (Fig. 2A). The EC50 and Emax values were 750 ± 238 nM and 55.8 ± 2.6% (n = 5), respectively (Fig. 2D). However, as shown in Fig. 2, A and C, the responses to carbachol were transient, and the amplitude of the EFS-induced contraction recovered to control levels within 7 to 10 min. Emax values (maximally inhibited contractions) and the magnitudes of contractions at 15 min after application of carbachol (1–30 μM) were 76.6 ± 3.1 and 93 ± 6.7% (n = 5) at 1 μM, 56.3 ± 6.1 and 83 ± 3.1% (n = 5) at 3 μM, 44.2 ± 2.6 and 80.3 ± 8.5% (n = 5) at 10 μM, and 51 ± 2.5 and 93 ± 2.4% (n = 5) at 30 μM, respectively (Fig. 2C). Because carbachol-induced inhibition of contractile responses reached its maximum within 3 min, concentration-response curves for the negative inotropic actions of carbachol were also constructed using the results obtained from cumulative carbachol application at 3-min intervals (Fig. 2, B and D). The concentration-inhibition relationships were virtually identical at carbachol concentrations below 1 μM, but the concentration-response curve obtained after cumulative carbachol application was not sigmoid but V-shaped at higher concentrations, probably due to slowly developed positive inotropic actions (3–30 μM) (Fig. 2D).
Fig. 2.
Effects of carbachol on EFS-induced contractions of left atria from wild-type (DDY) mice. A, typical traces of inotropic responses to 10 nM, 100 nM, 1 μM, 10 μM, and 100 μM carbachol applied noncumulatively. B, typical traces of inotropic response to carbachol applied cumulatively at 3-min intervals (10 nM, 100 nM, 1 μM, 10 μM, and 100 μM). C, time course of inotropic responses to carbachol (300 nM, 1 μM, 3 μM, 10 μM, and 30 μM). Ordinate, relative amplitude of EFS-induced contraction expressed as a percentage of the amplitude just before application of carbachol. Abscissa, incubation time with carbachol. D, comparison of concentration response curves for carbachol constructed from cumulative (▪) and noncumulative application of carbachol (•). Ordinate, relative amplitude of EFS-induced contraction expressed as a percentage of the amplitude just before application of carbachol. Abscissa, concentration of carbachol (log M). Values are means and S.E.M. of five to six experiments.
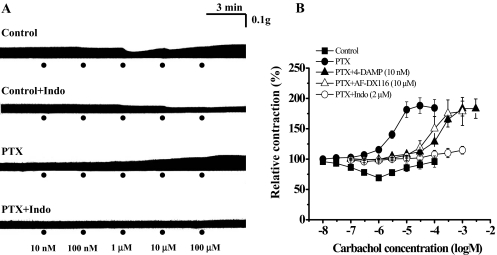
Strikingly, in left atria isolated from pertussis toxin-treated wild-type (DDY) mice, carbachol (1 μM–100 μM) increased the amplitude of EFS-induced contractions in a concentration-dependent manner (Fig. 3A). The carbachol EC50 and Emax values were 3.3 ± 0.9 μM and 184 ± 13.9% (n = 11), respectively (Fig. 3). Because the positive inotropic activity of carbachol was atropine-sensitive (100 nM; data not shown), the muscarinic receptor subtype involved in this activity was initially characterized by the use of three muscarinic receptor subtype-preferring antagonists: 4-DAMP (10 nM), AF-DX116 (10 μM), and p-F-HHSiD (1 μM). All three antagonists inhibited the positive inotropic action of carbachol, shifting carbachol concentration-response curves to the right in a parallel manner without change in Emax values. The estimated pKb values were 9.66 ± 0.21 (n = 4) for 4-DAMP, 6.28 ± 0.07 (n = 5) for AF-DX116, and 7.74 ± 0.16 (n = 4) for p-F-HHSiD (Fig. 3B). These pKb values were consistent with those for affinity to M3 muscarinic receptor (4-DAMP, 8.9–9.3; AF-DX116, 5.9–6.6; p-F-HHSiD, 7.8–8.2) (Dhein et al., 2001).
Fig. 3.
Inotropic responses to carbachol in left atria of pertussis toxin-treated wild-type (DDY) mice. A, typical inotropic responses to carbachol in left atria of control and pertussis toxin (PTX) (300 μg/kg i.p., 96-h)-treated wild-type mice. In atria of pertussis toxin-treated mice, the biphasic inotropic response pattern was changed to a positive inotropic response. In both control and pertussis toxin-treated mice, the positive inotropic responses to carbachol were decreased by indomethacin (2 μM; 30 min). B, concentration-inotropic response relationships of carbachol in electrically stimulated left atria of control (▪) and pertussis toxin-treated mice (•). 4-DAMP (10 nM; ▴) and AF-DX116 (10 μM; ▵) competitively antagonized the positive inotropic responses to carbachol. In atria from pertussis toxin-treated mice, indomethacin (2 μM; 30 min; ○) virtually abolished the positive inotropic responses to carbachol. In all experiments, carbachol was applied cumulatively at 3-min intervals. Ordinate, relative amplitude of EFS-induced contraction expressed as a percentage of the contraction just before application of carbachol. Abscissa, concentration of carbachol (log M). Symbols and vertical bars are means and S.E.M. of 4 to 11 experiments.
The positive inotropic responses to carbachol observed with atria from pertussis toxin-treated mice were nearly abolished by indomethacin (2 μM for 30 min) (Fig. 3). Indomethacin (2 μM for 30 min) also decreased the positive inotropic activity of carbachol in atria from nonpertussis toxin-treated control mice, and the biphasic inotropic responses were changed to a monophasic negative inotropic response (Fig. 3A).
Chronotropic and Inotropic Effects of Carbachol on Atria from Muscarinic Receptor KO Mice. The effects of carbachol on spontaneously beating right atria were compared between atria obtained from wild-type, M2KO, M3KO, and M2/M3KO mice. Spontaneous atrial beating frequencies (beats/min) were similar for wild-type mice (378 ± 22; n = 5) and muscarinic receptor KO mice (M2KO: 371 ± 36, n = 7; M3KO: 420 ± 32, n = 5; and M2/M3KO: 385 ± 35, n = 5). Carbachol concentration-dependently decreased spontaneous beating frequency of atria from wild-type and M3KO mice and finally abolished spontaneous beating at 10 to 30 μM (Fig. 4). The carbachol EC50 values were 1.4 ± 0.53 μM(n = 5) for wild-type and 1.2 ± 0.39 μM(n = 5) for M3KO preparations, respectively. Conversely, in atria from M2KO mice, the negative chronotropic actions of carbachol were abolished. Carbachol seemed to slightly increase heart rate at high concentrations (30–100 μM), but this effect was not statistically significant (Fig. 4). Carbachol had no effect at all on the spontaneous beating frequency of right atria from M2/M3KO mice (Fig. 4).
Fig. 4.
Comparison of chronotropic responses to carbachol in isolated right atria from wild-type and muscarinic receptor KO mice (M2KO, M3KO and M2/M3KO). Heart rates (beats/min) of isolated atria were 378 ± 22 (wild-type; n = 5), 371 ± 36 (M2KO; n = 7), 420 ± 32 (M3KO; n = 5), and 385 ± 35 (M2/M3KO; n = 5). Increasing concentrations of carbachol (10 nM, 30 nM, 100 nM, 300 nM, 1 μM, 3 μM, 10 μM, 30 μM, and 100 μM) were applied cumulatively at 3-min intervals, and concentration-response curves were constructed. Ordinate, heart rate expressed as percentage of baseline just before application of carbachol. Abscissa, concentration of carbachol (log M). Symbols and vertical bars are means and S.E.M. of five to seven experiments.
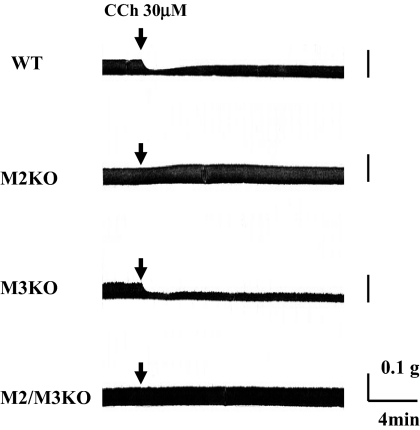
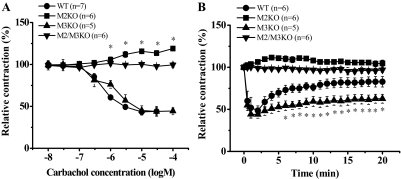
We next compared the inotropic responses to carbachol in left atria from wild-type and muscarinic receptor KO mice. Carbachol inhibited the EFS-induced contraction of wild-type atria in a concentration-dependent manner, but the inhibition was transient, as observed with atrial preparation from DDY mice (Figs. 5 and 6A). The EC50 and maximal inhibition were 700 ± 110 nM and 58 ± 3.4% (n = 6), respectively, when carbachol was applied in a noncumulative manner (Figs. 5 and 6A). In isolated atria from M3KO mice, carbachol caused negative inotropic actions with similar potency and efficacy as observed with wild-type atria (EC50 and maximal inhibition: 1.5 ± 0.5 μM and 56 ± 3.7%, respectively; n = 5) (Fig. 6A). However, in atria from M3KO mice, the inhibition was not transient but sustained (Figs. 5 and 6B).
Fig. 5.
Typical traces of inotropic responses to carbachol in left atria isolated from wild-type (WT), M2KO, M3KO, and M2/M3KO mice. After establishment of steady EFS-induced contractions, carbachol (30 μM) was applied as indicated by arrows.
Fig. 6.
Comparison of inotropic responses to carbachol in left atria isolated from wild-type (WT), M2KO, M3KO, and M2/M3KO mice. A, after establishing reproducible EFS-induced atrial contractions, carbachol (10 nM–100 μM) was applied noncumulatively at 30-min intervals, and concentration-response relationships were constructed. The asterisk (*) indicates significant increase compared with the values before application of carbachol. B, time courses of inotropic responses to carbachol in left atria isolated from WT, M2KO, M3KO, and M2/M3KO mice. After stable EFS-induced contractions had been obtained, carbachol (30 μM) was applied to the organ bath, and inotropic responses were observed for 20 min. Ordinate, relative amplitude of EFS-induced contraction expressed as a percentage of the amplitude just before application of carbachol. Abscissa, concentration of carbachol (log M) (A) and incubation time with 30 μM carbachol (min) (B). Values are means and S.E.M. of five to seven experiments. B, the asterisk (*) indicates significantly different compared with WT values.
Although the maximal inhibition by carbachol (30 μM) was almost the same in wild-type and M3KO atria (minimum amplitude of EFS-induced contractions: 46 ± 3.9% for wild-type mice, n = 6; 45 ± 5.5% for M3KO mice, n = 5; p = 0.95), the relative amplitudes of contraction were significantly reduced in M3KO atria at 5, 10, 15, and 20 min after application of carbachol (Fig. 6B). The following values were obtained in wild-type and M3KO atria: 63.1 ± 5.1 and 50 ± 4.5% (5 min; p = 0.09), 73 ± 6.0 and 54 ± 4.2% (10 min; p = 0.028), 72.4 ± 5.4 and 56 ± 4.5% (15 min; p = 0.038), and 75 ± 5.6 and 55 ± 5.5% (20 min; p = 0.04), respectively (Fig. 6B). Alternatively, carbachol did not decrease the EFS-induced contraction in atria from M2KO mice but significantly increased the EFS-induced contraction in a concentration-dependent manner (EC50 = 3.7 ± 1.6 μM; maximal response obtained at 100 μM, 119 ± 2.1%; n = 5) (Fig. 6A). This positive inotropic action reached a peak within 3 to 5 min after carbachol application and then declined slowly (Fig. 6B). Carbachol had no effect at all on the EFS-induced contraction of atria from M2/M3KO mice (Figs. 5 and 6).
Discussion
It is well documented that muscarinic agonists can induce both negative and positive inotropic responses in cardiac atria (Eglen et al., 1988; Du et al., 1995; Nishimaru et al., 2000; Tanaka et a., 2001). Overwhelming evidence indicates that the negative inotropic effects are mediated by the M2 muscarinic receptor subtype (Caulfield and Birdsall, 1998; Dhein et al., 2001). However, the nature of the muscarinic receptor subtype(s) mediating the positive inotropic activity of muscarinic agonists is a matter of argument and remains to be identified.
We demonstrated, by using real-time qRT-PCR expression analysis, that the M2 receptor mRNA is the predominant muscarinic receptor transcript found in mouse atria and ventricles but that the M1, M3, M4, and M5 receptor subtypes are also expressed at low levels in these tissues. This observation is consistent with the outcome of several previously published studies (Hardouin et al., 1998; Hellgren et al., 2000; Krejcí and Tucek, 2002; Wang et al., 2001; Pérez et al., 2006). Functional studies with right atria prepared from different muscarinic receptor knockout mice, complemented by studies with M2 muscarinic receptor subtype-preferring antagonists, indicated that muscarinic agonist-induced reduction in heart rate is mediated by the M2 receptor, in agreement with previous results (Caulfield and Birdsall, 1998; Stengel et al., 2000; Dhein et al., 2001). In the present study, carbachol was unable to produce statistically significant positive chronotropic responses in right atria from M2KO mice and pertussis toxin-treated mice. These results suggest that the M3 receptor lacks chronotropic activity in right atria of mice under the experimental conditions used (360–380 beats/min).
In left atria from wild-type mice, carbachol inhibited EFS-induced contractions followed by a gradual increase in contraction amplitude. After in vivo pertussis toxin treatment of wild-type mice, carbachol did not show any negative inotropic activity in atrial preparations but only positive inotropic responses. This observation indicated that the positive inotropic effects of carbachol are mediated by either M1, M3, or M5 receptors (or a combination of two or all of these receptors), which couple to pertussis toxin-insensitive G proteins (G proteins of the Gq/11 family). Studies with muscarinic receptor subtype-preferring antagonists suggested that the positive inotropic effects of muscarinic agonists in mice may be mediated by the M3 receptor (Nishimaru et al., 2000; Tanaka et al., 2001). However, the antagonists used in these studies show a rather limited degree of receptor subtype selectivity (M3 and M5 receptors, e.g., show a very similar ligand binding pharmacology; Caulfield and Birdsall, 1998). Moreover, as indicated above, transcripts for all five muscarinic receptor subtypes are found in mouse atria, further complicating the interpretation of classical pharmacological studies of the roles of non-M2 muscarinic receptor subtypes in atrial function.
To identify the molecular nature of the muscarinic receptor subtype mediating the positive inotropic effects of muscarinic agonist, we used muscarinic receptor knockout mice as novel experimental tools (Wess, 2004). In atria from M3KO mice, the maximal negative inotropic activity of carbachol was comparable with that found with wild-type atria. However, the time course of carbachol-mediated inotropic effects was different in atria from M3KO mice. The slow increase in EFS-induced contractions observed in atria from wild-type mice was significantly decreased in atria from M3KO mice. The biphasic inotropic effects of carbachol seen with wild-type atria were changed to an almost monophasic sustained negative inotropic response in M3KO atria. Conversely, in atria from M2KO mice, carbachol was devoid of negative inotropic activity and only induced positive inotropic effects. In atria from M2/M3 double KO mice, carbachol had neither positive nor negative inotropic effects. These data clearly indicate that the M2 receptor mediates the negative inotropic action and that the M3 receptor mediates the positive inotropic effects of carbachol in mouse atria. One possible mechanism for the observed transient negative inotropic action of carbachol in wild-type atria may involve M2 receptor desensitization. However, in M3KO atria, the negative inotropic action of carbachol was sustained for 20 min, suggesting that M2 receptor desensitization does not play a significant role under the experimental conditions used in this study.
At present, it remains unclear whether the M3 muscarinic receptors mediating the positive inotropic effects of muscarinic agonists are localized on cardiomyocytes or on other cardiac cell types such as neurons or endothelial cells (note that the samples for real-time qRT-PCR were obtained from whole atria or ventricles in the present study). An immunohistochemical study indicated that M3 receptors are present on cardiomyocytes (Wang et al., 2001). In addition, M3 receptor-mediated increases in inositol phosphate formation have been observed with dispersed cardiomyocytes (Pönicke et al., 2003; Myslivecek et al., 2008). Alternatively, because M3 receptor-mediated positive inotropic effects were abolished by treatment with 1% Triton X-100 or indomethacin, it has been proposed that M3 receptors present on the endocardial endothelium stimulate the production of endogenous prostaglandins eliciting positive inotropic actions (Tanaka et al., 2001). Hassall et al. (1993) reported the expression of M1 to M4 receptor mRNA in intrinsic neurons of rat and guinea pig hearts. In the present study, we demonstrated that indomethacin inhibited the positive inotropic actions of carbachol in atria from pertussis toxin-treated mice, in agreement with a previous report (Tanaka et al., 2001). Clearly, however, more detailed studies are needed to clarify the precise localization of the M3 muscarinic receptors meditating the positive inotropic actions in the mouse atrium.
Muscarinic receptor agonists are known to cause positive inotropic effects in several species (rat, guinea pig, mouse, human, and chicken). It has been demonstrated that the M1 receptor can stimulate L-type Ca2+ currents in guinea pig ventricular cells (Gallo et al., 1993) and Ca2+ release in rat ventricular cells (Sharma et al., 1996), causing positive inotropic effects. The M1 receptor has also been implicated in mediating positive inotropic responses in isolated human (Du et al., 1995) and chicken myocardium (Nouchi et al., 2007). Conversely, Protas et al. (1998) reported that the M2 receptor, the same receptor subtype mediating inhibition of cardiac contractility, caused positive inotropic effects through activation of Na+-Ca2+ exchange and myofilament Ca2+ sensitivity in guinea pigs. In the present study, we clearly demonstrated that the M3 receptor mediates the positive inotropic action of carbachol in mouse atria. Taken together, these findings suggest that the molecular nature of the muscarinic receptor subtypes mediating cardiac positive inotropic effects and the underlying mechanisms may differ from species to species.
In conclusion, consistent with previous studies, we found that the negative chronotropic and negative inotropic actions of muscarinic receptor agonists in mouse atria are mediated by the M2 muscarinic receptor. In addition, we provide unambiguous evidence, by using atria prepared from muscarinic receptor knockout mice, that the positive inotropic actions of muscarinic agonists are mediated by the M3 muscarinic receptor subtype. We speculate that this activity serves to counteract or dampen the inhibitory inotropic effects observed after M2 receptor activation in cardiac atria.
This research was supported by the Intramural Research Program of the National Institutes of Health [National Institute of Diabetes and Digestive and Kidney Diseases].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.153304.
ABBREVIATIONS: M2KO, M2 receptor single knockout; M3KO, M3 receptor single knockout; M2/M3KO, M2 and M3 receptors double knockout; qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction; PCR, polymerase chain reaction; EFS, electrical field stimulation; AF-DX116, 11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one; 4-DAMP, 4-diphenylacetoxy-N-methyl-piperidine; p-F-HHSiD, p-fluorohexahydrosiladiphenidol hydrochloride.
References
- Brodde OE and Michel MC (1999) Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev 51 651-690. [PubMed] [Google Scholar]
- Caulfield MP and Birdsall NJ (1998) International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50 279-290. [PubMed] [Google Scholar]
- Dhein S, van Koppen CJ, and Brodde OE (2001) Muscarinic receptors in the mammalian heart. Pharmacol Res 44 161-182. [DOI] [PubMed] [Google Scholar]
- Du XY, Schoemaker RG, Bos E, and Saxena PR (1995) Characterization of the positive and negative inotropic effects of acetylcholine in the human myocardium. Eur J Pharmacol 284 119-127. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Montgomery WW, and Whiting RL (1988) Negative and positive inotropic responses to muscarinic agonists in guinea pig and rat atria in vitro. J Pharmacol Exp Ther 247 911-917. [PubMed] [Google Scholar]
- Gallo MP, Alloatti G, Eva C, Oberto A, and Levi RC (1993) M1 muscarinic receptors increase calcium current and phosphoinositide turnover in guinea-pig ventricular cardiocytes. J Physiol 471 41-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, and Wess J (1999) Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 96 1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardouin S, Bourgeois F, Toraasson M, Oubenaissa A, Elalouf JM, Fellmann D, Dakhli T, Swynghedauw B, and Moalic JM (1998) beta-Adrenergic and muscarinic receptor mRNA accumulation in the sinoatrial node area of adult and senescent rat hearts. Mech Ageing Dev 100 277-297. [DOI] [PubMed] [Google Scholar]
- Hassall CJ, Stanford SC, Burnstock G, and Buckley NJ (1993) Co-expression of four muscarinic receptor genes by the intrinsic neurons of the rat and guinea-pig heart. Neuroscience 56 1041-1048. [DOI] [PubMed] [Google Scholar]
- Hellgren I, Mustafa A, Riazi M, Suliman I, Sylvén C, and Adem A (2000) Muscarinic M3 receptor subtype gene expression in the human heart. Cell Mol Life Sci 57 175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejcí A and Tucek S (2002) Quantitation of mRNAs for M1 to M5 subtypes of muscarinic receptors in rat heart and brain cortex. Mol Pharmacol 61 1267-1272. [DOI] [PubMed] [Google Scholar]
- Lanzafame AA, Christopoulos A, and Mitchelson F (2003) Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors Channels 9 241-260. [PubMed] [Google Scholar]
- Myslivecek J, Klein M, Novakova M, and Ricny J (2008) The detection of the non-M2 muscarinic receptor subtype in the rat heart atria and ventricles. Naunyn Schmiedebergs Arch Pharmacol 378 103-116. [DOI] [PubMed] [Google Scholar]
- Nishimaru K, Tanaka Y, Tanaka H, and Shigenobu K (2000) Positive and negative inotropic effects of muscarinic receptor stimulation in mouse left atria. Life Sci 66 607-615. [DOI] [PubMed] [Google Scholar]
- Nouchi H, Kaeriyama S, Muramatsu A, Sato M, Hirose K, Shimizu N, Tanaka H, and Shigenobu K (2007) Muscarinic receptor subtypes mediating positive and negative inotropy in the D developing chick ventricle. J Pharmacol Sci 103 75-82. [DOI] [PubMed] [Google Scholar]
- Pérez CC, Tobar ID, Jiménez E, Castañeda D, Rivero MB, Concepción JL, Chiurillo MA, and Bonfante-Cabarcas R (2006) Kinetic and molecular evidences that human cardiac muscle express non-M2 muscarinic receptor subtypes that are able to interact themselves. Pharmacol Res 54 345-355. [DOI] [PubMed] [Google Scholar]
- Pönicke K, Heinroth-Hoffmann I, and Brodde OE (2003) Demonstration of functional M3-muscarinic receptors in ventricular cardiomyocytes of adult rats. Br J Pharmacol 138 156-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protas L, Shen JB, and Pappano AJ (1998) Carbachol increases contractions and intracellular Ca++ transients in guinea pig ventricular myocytes. J Pharmacol Exp Ther 284 66-74. [PubMed] [Google Scholar]
- Sharma VK, Colecraft HM, Wang DX, Levey AI, Grigorenko EV, Yeh HH, and Sheu SS (1996) Molecular and functional identification of m1 muscarinic acetylcholine receptors in rat ventricular myocytes. Circ Res 79 86-93. [DOI] [PubMed] [Google Scholar]
- Stengel PW, Gomeza J, Wess J, and Cohen ML (2000) M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther 292 877-885. [PubMed] [Google Scholar]
- Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, Wess J, and Haberberger RV (2003) Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol 64 1444-1451. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Nishimaru K, Kobayashi M, Matsuda T, Tanaka Y, and Shigenobu K (2001) Acetylcholine-induced positive inotropy mediated by prostaglandin released from endocardial endothelium in mouse left atrium. Naunyn Schmiedebergs Arch Pharmacol 363 577-582. [DOI] [PubMed] [Google Scholar]
- Wang H, Han H, Zhang L, Shi H, Schram G, Nattel S, and Wang Z (2001) Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol Pharmacol 59 1029-1036. [DOI] [PubMed] [Google Scholar]
- Wang Z, Shi H, and Wang H (2004) Functional M3 muscarinic acetylcholine receptor in mammalian hearts. Br J Pharmacol 142 395-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J (2004) Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol 44 423-450. [DOI] [PubMed] [Google Scholar]
- Willmy-Matthes P, Leineweber K, Wangemann T, Silber RE, and Brodde OE (2003) Existence of functional M3-muscarinic receptors in the human heart. Naunyn Schmiedebergs Arch Pharmacol 368 316-319. [DOI] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, and Kurachi Y (1998) G protein regulation of potassium ion channels. Pharmacol Rev 50 723-760. [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, et al. (2001) Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 410 207-212. [DOI] [PubMed] [Google Scholar]