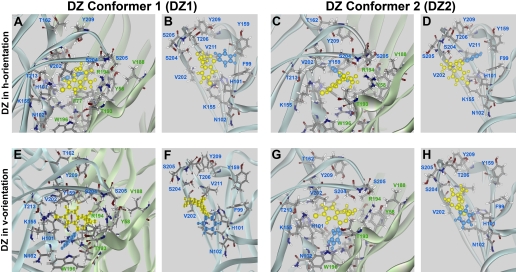
Fig. 6.
Orientations of DZ were modeled after orientations of the DZ-NCS. The methyl substituent at the N1 atom of DZ is much smaller in size than the polymethyl linker used for affinity purification (Fig. 9) and is not able to restrict the ability of the benzodiazepine ring to undergo inversions, making it more difficult to deduce which of the two conformers is likely to be prevalent in the binding pocket. DZ's seven-member benzodiazepine ring is yellow, and the C5-phenyl is blue. A and B, DZ1h; C and D, DZ2h; E and F, DZ1v; G and H, DZ2v orientation. In all of these models, the C7-chloro group is directed toward the α1His101 and α1Lys155 residues, and the C2 carbonyl group is located in close proximity to α1Ser204, α1Ser205, γ2Thr193, and γ2Arg194, where it is able to make hydrogen bonds. Pairs of images depict the BZD binding pocket viewed from outside of the receptor (A, C, E, and G) and from within the binding pocket looking toward the α subunit (B, D, F, and H).