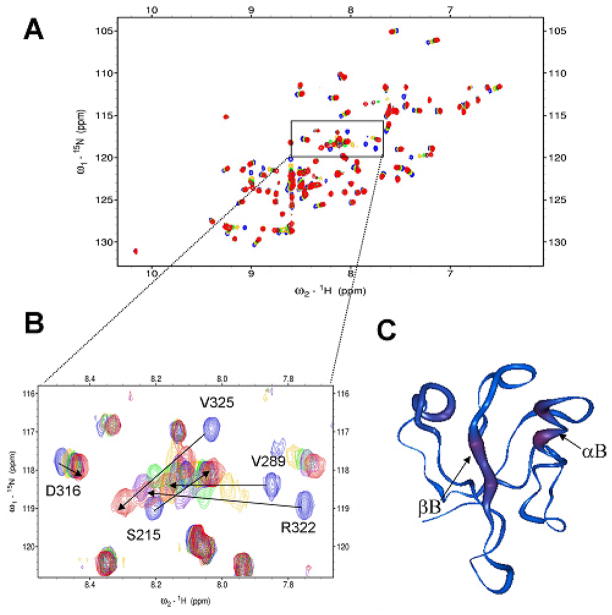
Figure 1.
Tripeptide VVV interacts with the Dvl1 PDZ domain. (a) 15N-HSQC spectra of 15N-labeled Dvl1 PDZ domain alone (0.3 mM, blue) and with varying concentrations of tripeptide VVV. (b) The extended 15N-HSQC spectra of the Dvl1 PDZ domain as a function of the tripeptide VVV concentration (blue: free, gold 1:4; green: 1:7; purple 1:11, red 1:22). (c) The worm representation of the backbone structure of the Dvl1 PDZ domain. The thickness and color of the worm is proportional to the weighted sum of the proton and amide chemical shift perturbations upon binding of peptide VVV (red, high; blue, low). The program Insight II was used was used to prepare Fig. 1c.