Abstract
Objective
Recent studies have demonstrated efficacy of targeted therapy combined with radiotherapy in HNSCC. We hypothesized that a combination treatment including a replicating adenovirus armed with tissue inhibitor metalloproteinase-2 (TIMP-2), radiation, and Cisplatin will augment treatment response and reduce tumor growth in vivo of HNSCC xenografts.
Design
Both single-agent (TIMP-2 virus, radiation, and Cisplatin) and the combination therapies were evaluated in vitro and in vivo. The efficacy of both single agent and combination therapies in vivo was determined by monitoring of tumor growth and immunohistochemistry.
Results
Treatment with replicative Ad-TIMP-2 virus and radiation decreased cell viability in vitro and resulted in an additional anti-angiogenic response in vivo. Tumor response rates to treatment with replicative Ad-TIMP-2, radiation, Cisplatin, or combination therapies ranged from limited inhibition of tumor growth of the single-agent therapy to a statistically significant additive anti-tumor response with the combination therapies. Replicative Ad-TIMP-2 + radiation + Cisplatin in the SCC1 nude mice demonstrated the greatest response rates in tumor growth and angiogenesis.
Conclusions
Combination Ad-TIMP-2 gene therapy with radiation and the triple treatment group resulted in an augmented therapeutic response. This is the first report of the potential benefits of combining radiation and MMP inhibitor treatment.
Keywords: TIMP-2, chemoradiation, gene therapy, HNSCC
INTRODUCTION
With more than 780,000 new cases diagnosed annually worldwide, head and neck squamous cell carcinoma (HNSCC) is the fifth most common cancer [1]. Advanced HNSCC treatment, which may include surgery with radiotherapy and/or chemoradiation, demonstrates limited success and no significant improvement in the 5-year survival [2, 3]. Replicative adenovirus has shown promise for HNSCC gene therapy, in particular ONYX-015 tumor/tissue-restricted replicative adenoviruses [4]. The initial viral infection of the target tumor cells can produce progeny virions that infect adjacent cancer cells, thereby improving in vivo infectivity, intratumor distribution, and bystander effects mediated by adenovirus. However, tumor/tissue-restricted replicative adenoviruses exhibited only limited therapeutic efficacy in clinical trials when used as a monotherapy [5]. To improve the efficacy of tumor/tissue-restricted replicative adenoviruses, they need to be combined with other therapeutic agents.
The major roles of matrix metalloproteinases (MMPs) are homeostatic regulation of the extracellular environment and controlling innate immunity [6]. The MMPs are associated with cancer cell growth and angiogenesis, basement membrane destruction, and evasion of apoptosis [7, 8]. The tissue inhibitors of metalloproteinases (TIMPs) are expressed in multiple cell types and inhibit the catalytic activation of many pro-MMPs. The TIMPs participate in complex biologic functions that extend beyond their roles as inhibitors of MMP activity; for example, they induce changes in cell morphology, stimulate growth of several cell types, promote germ cell development, and inhibit angiogenesis [9, 10].
Although there is a strong biological basis for the development of strategies employing MMP inhibitors, the clinical response rates of MMP inhibitors as single-agents in cancer therapy trials have been modest at best [11, 12]. One possible explanation for these disappointing results might be that MMPs are involved at early stages of tumor progression, whereas clinical trials are generally restricted to advanced-stage disease. Studies in tumor xenograft models have suggested that the best potential of anti-MMPs might be displayed in treatments that combine MMP inhibitors with conventional therapeutic strategies [13].
The TIMPs occur naturally within the extracellular matrix. MMP-2 over-expression has been shown to enhance metastasis of HNSCC, while TIMP-2 has been shown to suppress cancer metastasis in patients [14]. TIMP-2 reduces the invasion of malignant cells through membranes in vitro, limits the propagation of tumors in vivo, inhibits angiogenesis in an MMP independent manner, and inhibits the release of latent growth factors in the extracellular matrix [15–17].
The TIMPs are naturally produced and are both anti-proliferative and anti-angiogenic, but are cytostatic and not cytotoxic. Other targeted molecular therapies have been found to be more effective in combination with cytotoxic treatment [18]. Previous work using MMP based inhibitors for treatment of HNSCC has exclusively focused on the use of single agents.
TIMP-2 acts on a broad panel of MMPs, and so an accumulative benefit could be expected as they simultaneously target tumor cells, subsequent metastasis, tumor growth, and angiogenesis. The anti-tumor efficacy TIMPs has been demonstrated in many types of solid tumors in animal models [19, 20]. Anti-angiogenic therapy represents a promising approach to cancer treatment. The use of anti-angiogenic therapy to combat cancer has several advantages. First, angiogenesis is a phenomenon seen in all types of cancers. Second, the patient’s risk of resistance to angiostatic agents seems low, compared with those associated with the use of cytotoxic drugs alone [21]. Third, the side effects of cytostatic/angiostatic agents are low and therefore these agents can be used in combination with multiple other therapies. For the therapy to be effective, it must continue for a prolonged period such that the tumor becomes dormant. As a result, gene therapy is a strategy worth exploring because, once perfected, it could produce a long-term effect.
The use of conditionally replicative adenoviruses (CRAds) for cancer gene therapy has been explored and despite some encouraging results in clinical trials, their use as single agent therapy has demonstrated limitations in terms of complete tumor eradication or sustained antitumor response. The combination of CRAds with chemotherapy agents has been found to enhance their anti-tumor efficacy [22].
In this study, we used a combination treatment of replicative adenovirus encoding TIMP-2 cDNA along with radiation and chemotherapy for maximal therapeutic effects in a HNSCC xenograft model. We postulate that replicative adenovirus will augment the cytolytic effect and produce increased expression of TIMP-2 protein, thus inhibiting tumor growth and angiogenesis; while the cytotoxic agents, radiation and Cisplatin, will increase the level of tumor cell killing. The primary aim of this research was to investigate the therapeutic effects of Ad-TIMP-2 gene therapy in combination with radiation therapy (XRT) and Cisplatin in a human HNSCC nude mouse xenograft model. Both single-agent (Ad-TIMP-2, Ad Wt 300, Ad-TIMP-2 + Ad Wt 300, XRT, Cisplatin) and the combination therapies (Ad-TIMP-2 + Ad Wt 300 + XRT, Ad-TIMP-2 + Ad Wt 300 + Cisplatin, XRT + Cisplatin, and Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin) were evaluated in vitro and in vivo. The efficacy of both single agent and combination therapies was determined by tumor growth inhibition, survival, TIMP-2 and MMP-2 protein expression, apoptosis, proliferation, and angiogenesis. This work may lead to a potential therapy for treatment of HNSCC using this combination therapy approach.
MATERIALS AND METHODS
CELL LINES AND REAGENTS
Human head and neck cell line, SCC1, was obtained from the American Type Culture Collection (Manassas, VA). SCC1 cells were grown in DMEM supplemented with L-glutamine and 10% FBS. For xenografts, cells were initially injected into Athymic, nude mice to grow for future xenografts into mice for in vivo studies. Cisplatin was purchased from Sigma as a 1mg/ml stock solution. For animal studies, Cisplatin was diluted in 0.9% saline immediately before injection.
ADENOVIRUS
Ad Wt 300, a wild-type human adenovirus serotype 5, was obtained from ATCC. Ad-TIMP-2 was a gift from Dr. J. Douglas (University of Alabama at Birmingham). It has been previously described [23] and is an E1, E3-deleted replication deficient Adenovirus serotype 5 vector that expresses human TIMP-2 under the control of the CMV promoter. Both Ad Wt 300 and Ad-TIMP-2 were propagated in 293 cells and purified using cesium chloride centrifugation. The viral particle concentration was measured at 260 nm in which absorbance of 1 corresponds to 1.1 × 1012 particles/ml. When a non-replicative Ad armed with a therapeutic protein and wild type Ad are added simultaneously, a replicative Ad virus is produced which will generate more of the therapeutic protein than the non-replicative Ad alone [24].
TIMP-2 levels in Ad-TIMP-2 infected SCC1 cells
Monolayers of SCC1 cells, were trypsinized and resuspended in complete culture medium in a 6-well plate 24 hr prior to treatment. Cells were treated with PBS, Ad-TIMP-2 MOI 1 alone, or Ad-TIMP-2 + Ad Wt 300 MOI 1. After 48 h, media and cells were collected separately for Western blot. Protein levels were compared using actin from the cell lysate.
WESTERN BLOTTING
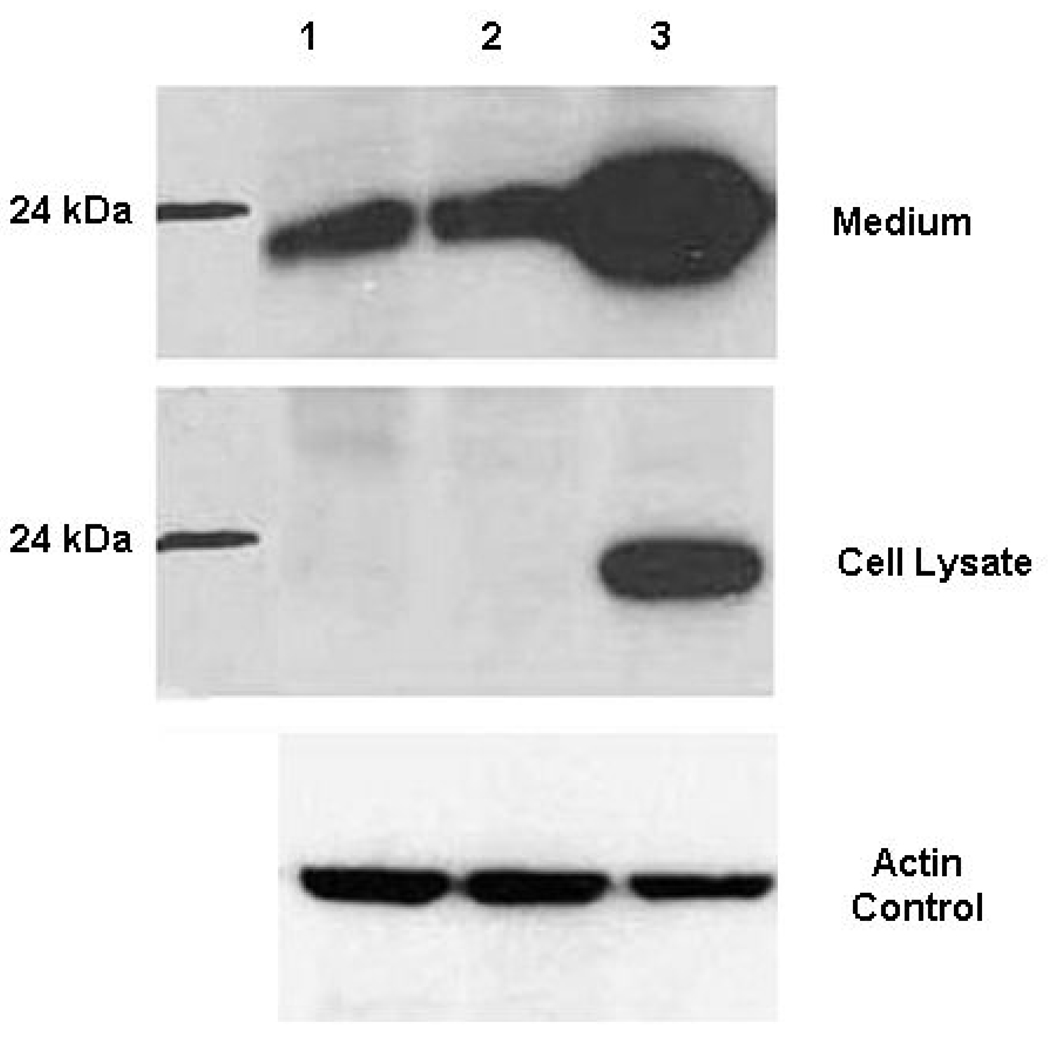
After infection with Ad-TIMP-2 or Ad-TIMP-2 +Ad Wt 300, SCC1 cells were incubated for 48 h and then media was collected for the Western blot. Supernatant (20 µl) was loaded onto 12.5% SDS-page gel electrophoresis and electrotransferred onto a nitrocellulose membrane. The transferred membranes were blocked in 5% nonfat milk for 1 h at room temperature, followed by incubation with primary antibody for 1 h at room temperature. After washing three times with TTBS (0.02 M Tris–HCl buffer, pH 7.5, 0.137 M NaCl, and 0.1% Tween 20), the membranes were incubated with horseradish peroxidase-conjugated (secondary antibody name dilution). Detection by the chemiluminescence reaction was carried out using a chemiluminescence kit followed by exposure to X-ray film (Fig. 1).
Figure 1. Western blot analysis of TIMP-2 expression.
SCC-1 cells were infected with Ad-TIMP-2 MOI 1 or Ad-TIMP-2 + Ad Wt 300 MOI 1. After 48 h, media and cells were collected separately for Western blot. Protein levels were compared using actin from the cell lysate. Lane 1 PBS, Lane 2 Ad-TIMP-2 MOI 1, Lane 3 Ad-TIMP-2 + Ad Wt 300 MOI 1.
CELL VIABILITY ASSAY
Monolayers of SCC1 cells, were trypsinized and resuspended in complete culture medium. SCC1 cells were plated in duplicate in 96-well plates. The sequence and timing of each experiment was identical. Cells were then incubated at 37°C in an atmosphere of 5% CO2 for 24 h prior to treatments. Virus and Cisplatin were diluted in culture medium immediately before use. Cells were treated with both single-agents and in combination of virus, radiation, and Cisplatin. Cells treated with combinations were treated with Cisplatin for 1 hr prior to treatment with radiation. Cells treated with combinations including virus were treated 4 h after radiation and/or Cisplatin. Cells were washed with PBS with 10 % FBS and fresh medium was added to cells subsequent to treatment. Cell viability was assessed after 48-h exposure to radiation, Cisplatin, or virus with Trypan Blue or ATPLite assay. All samples were assayed in quadruplicate and all experiments were repeated at least once. Cells were treated with 10 MOI or 100 MOI Ad-TIMP-2 with and without Ad Wt 300. To evaluate combination treatments in vitro, cells were treated with 4 Gy or 8 Gy radiation, 1 MOI Ad-TIMP-2 + Ad Wt 300, and/or 1 µM Cisplatin. Cells were collected at the indicated time. Experiments were repeated at least once. Cell viability comparisons were made between each single treatment, no treatment, and the combination treatments using a one-way ANOVA and Tukey with SAS.
COLLAGEN DEGRADATION ASSAY
The collagen degradation assay was performed as previously described [25]. A monolayer of SCC1 cells was infected with Ad-TIMP-2 alone, Ad-TIMP-2 + Ad Wt 300, or uninfected. After 48 h, conditioned media was removed from each set of infected cells and filtered with a 100,000 MW filter to remove virus. Collagen-coated wells, containing 0.65 mL per well of 300 µg/mL type I collagen (Becton Dickinson, Bedford, MA), were incubated at 37°C for 2 h and allowed to dry in a laminar flow hood for 24–48 h. SCC-1 cells were placed in the center of each well and allowed to adhere over 2 h at 37°C. Cultures were incubated for 4 days in conditioned media. Wells were washed and stained with Coomassie blue (0.2%), and the extent of collagenolysis in the center of the well where the cells were seeded was determined by imaging (Coolpix 4500, Nikon USA, Melville, NY). To determine the effect of inhibitors on collagen degradation, the presence of the inhibitors was maintained [26].
APOPTOSIS
SCC1 cells were plated in 6-well plates and incubated overnight at 37oC. Cells were treated with radiation (4 or 8 Gy). The cells were also treated with Ad-TIMP-2 + Ad Wt 300 (10 MOI) 4 h post-radiation. Apoptotic cells were quantified using the Annexin V-FITC Apoptosis detection kit according to the manufacturer’s instructions (Bio Vision). Approximately 5 × 105 cells were resuspended in 100 µl of manufacturer-supplied 1 × binding buffer, and mixed with 5 µl of Annexin V-FITC and 5 µl of PI. After 15 min incubation in the dark at room temperature, the cells were analyzed using FACS (Table 1). The percentage of apoptosis was compared between groups using a Student’s T-test with SAS software.
Table 1.
SCC1 cells were plated in 6-well plates and incubated overnight at 37°C. Cells were treated with radiation (4 or 8 Gy). The cells were also treated with Ad-TIMP-2 + Ad Wt 300 (10 MOI) 4 h post-radiation. Apoptosis was measured at 24 or 48 h post-radiation.
| Treatment | ||
|---|---|---|
| Radiation | PBS | Ad-TIMP-2 + Ad Wt 300 MOI 10 |
| 4 Gy (24 h) | 0.3a | 1.2 |
| 4 Gy (48 h) | 0.4 | 1.8 |
| 8 Gy (24 h) | 0.5 | 1.8 |
| 8 Gy (48 h) | 0.8 | 3.0 |
Percentage of Annexin-V FITC stained cells
IMMUNOHISTOCHEMISTRY AND WESTERN BLOT OF TUMORS
Three month-old female nude mice were inoculated with 2 mm2 established SCC1 tissue subcutaneously in the flank. Treatment started once the tumors were 4–6 mm in diameter. Mice were randomly divided into groups of 4 mice to receive different treatments: (1) Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin; (2)Ad-TIMP-2 + Ad Wt 300 + XRT; (3) Ad-TIMP-2 + Ad Wt 300 + Cisplatin; (4) Ad-TIMP-2 + Ad Wt 300; (5) XRT + Cisplatin; (6) Cisplatin; (7) Ad-TIMP-2 + Ad Wt 300 + Cisplatin; (8) control. Animals injected with virus received 1 × 108 particles in 30 µl of Ad-TIMP-2 and Ad Wt 300 with a microsyringe for three separate needle passes. Lead shielded animals received 8 Gy of 60Co radiation to the exposed tumor, divided into 2 Gy fractions on days 14, 19, 23, and 26. Animals received 4 intraperitoneal doses of Cisplatin (3 mg/kg) 1 h before each fraction of radiation. Animals were terminated 2 and 10 days after the end of treatment and tumors were harvested for immunohistochemistry analysis.
IMMUNOHISTOCHEMISTRY AND ANGIOGENESIS OF TUMORS
Tumor xenografts from treated animals were harvested and fixed into paraffin blocks. The primary polyclonal antibody AB 1055 (Chemicon International, Temecula, CA) is specific for hexon protein of adenovirus (Fig. 4). Angiogenesis was determined using a Factor VIII antibody (Sigma) (Fig. 5). Formalin-fixed, paraffin-embedded tumors were cut into 4 µm sections and put onto slides. After slides were deparaffinized and rehydrated, antigen retrieval was preformed in a pressure cooker for 20 min in pH 8 EDTA buffer. After H2O2 quench and 3% horse serum blocking, slides were incubated with the primary antibody at 1:1000 dilution for 20 min followed by Signet HRP label for 20 min and Biobenex DAB for 7 min. Slides were counterstained with hematoxylin H&E safranin staining was performed on all of the xenografts for morphology. Blood vessels were counted in treated tumor xenografts based on 3 images per treatment and a standard grid for quantification (Fig. 6). Angiogenesis was compared between groups using ANOVA with SAS software.
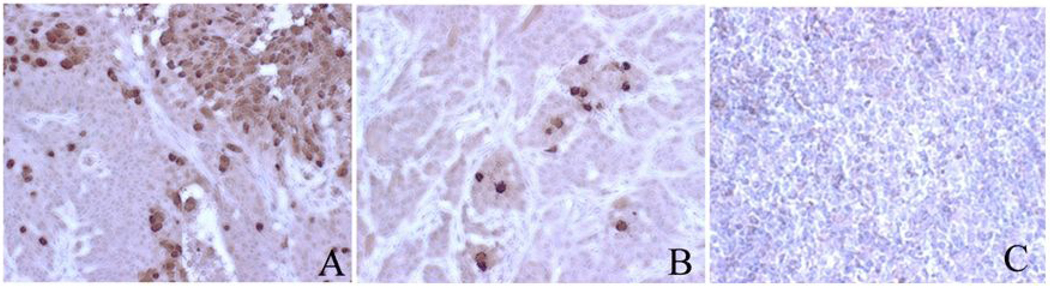
Figure 4. Immunohistochemistry staining for hexon protein in SCC-1 xenografts.
Tumors were excised from nude mice after 2 injections with Ad-TIMP-2 + Ad Wt 300. Cells containing the adenoviral hexon protein are stained brown (A and B), whereas no staining was observed in uninfected control tumor (C). High concentrations of virus were seen at injection points (A). Positive focal areas were seen dispersed throughout the tissue (B). Magnification 400X
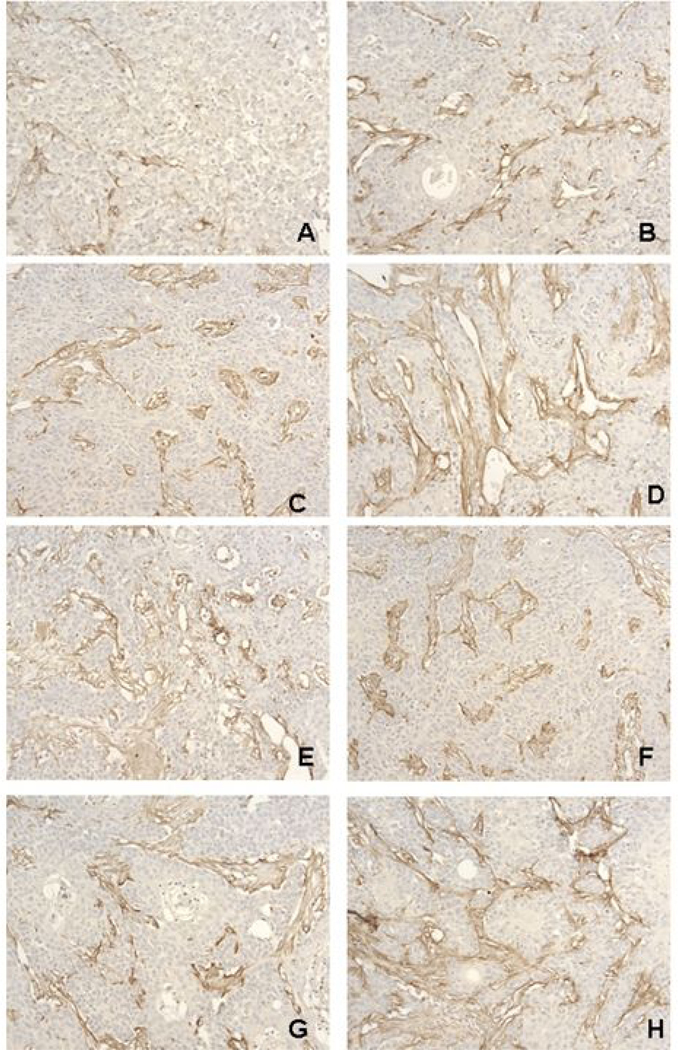
Figure 5. Immunohistochemistry staining for Factor VIII in SCC-1 xenografts.
Nude mice were inoculated with SCC-1 tumor tissue s.c. in the flank. Treatment started once the tumors were 6–8 mm in diameter. Mice were randomly divided into groups of 5 mice to receive different treatments: (A) Ad-TIMP-2/Ad Wt 300 + XRT + Cisplatin; (B) Ad-TIMP-2/Ad Wt 300 + XRT; (C) Ad-TIMP-2/Ad Wt 300; (D) XRT; (E) XRT + Cisplatin; (F) Cisplatin; (G) Ad-TIMP-2/Ad Wt 300 + Cisplatin; and (H) control. Animals injected with virus received 1 × 108 particles in 50 µl of Ad-TIMP-2 and Ad Wt 300 with a microsyringe for three separate needle passes. Animals receiving virus were injected intratumorally on day 2 and 8 after tumor implantation. Animals in the radiation group received 8 Gy of 60Co radiation to the tumor mass divided into 4 fractions on days 0, 4, 8 and 12. Animals received 4 intraperitoneal doses of Cisplatin (3 mg/kg) 1 h before each fraction of radiation. The Cisplatin regimen was previously established separately. Angiogenesis was determined using a Factor VIII antibody. Blood vessels are stained brown. The triple treatment group (Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin) had the fewest blood vessels. Each group which was treated with replicative Ad-TIMP-2 alone or in combination resulted in an anti-angiogenic effect in comparison to radiation alone, Cisplatin alone, radiation + Cisplatin, or untreated control. Magnification 400X.
Figure 6. Quantification of angiogenesis in SCC-1 xenografts.
Tumors were excised from nude mice at 2 and 10 days post-treatment. Treatment of tumors was as follows: (A) Ad-TIMP- 2 + Ad Wt 300 + XRT + Cisplatin; (B) Ad-TIMP-2 + Ad Wt 300 + XRT; (C) Ad-TIMP-2 + Ad Wt 300; (D) XRT; (E) XRT + Cisplatin; (F) Cisplatin; (G) Ad-TIMP-2 + Ad Wt 300 + Cisplatin; (H) untreated. Angiogenesis was determined using a Factor VIII antibody. Blood vessels were counted in 3 images per treatment using a standard grid for quantification. **indicates statistical significance P=0.002. The triple treatment group (Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin) had the fewest blood vessels. Each tumor was treated with replicative Ad-TIMP-2 alone or in combination resulted in an anti-angiogenic effect in comparison to radiation alone, Cisplatin alone, radiation + Cisplatin, or no treatment.
IN VIVO EFFICACY OF AD-TIMP-2 AND CHEMORADIATION
Three month-old female nude mice were inoculated using a trocar needle with 2 mm2 established SCC1 tissue subcutaneously in the flank. Treatment started once the tumors were 5–8 mm in diameter. Mice were randomly divided into groups of 7 mice to receive different treatments: (1) Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin; (2) Ad-TIMP-2 + Ad Wt 300 + XRT; (3) Ad-TIMP-2 + Ad Wt 300; (4) XRT; (5) XRT + Cisplatin; (6) Cisplatin; (7) Ad-TIMP-2 + Ad Wt 300 + Cisplatin; (8) Ad Wt 300; and (9) control. Animals injected with virus received 1 × 108 particles in 50 µl of Ad-TIMP-2 and Ad Wt 300 with a microsyringe for three separate needle passes. Animals receiving Ad-TIMP-2 + Ad Wt 300 virus were injected intratumoral on days 16 and 23 after tumor implantation with 1 × 108 particles in 30 µl of Ad-TIMP-2 and Ad Wt 300. Group 8 animals received Ad Wt 300 alone in the same manner as animals receiving virus. Lead shielded animals received 8 Gy of 60Co radiation to the exposed tumor, divided into 4 fractions on days 14, 19, 23, and 26. Animals received 4 intraperitoneal doses of Cisplatin (3 mg/kg) 1 h before each fraction of radiation. Tumors were measured biweekly for 2 months. Potential treatment toxicity was monitored using mouse weight. Tumor size (surface area equal to product of two largest diameters) and regression rates were determined in each treatment group (Fig. 7).
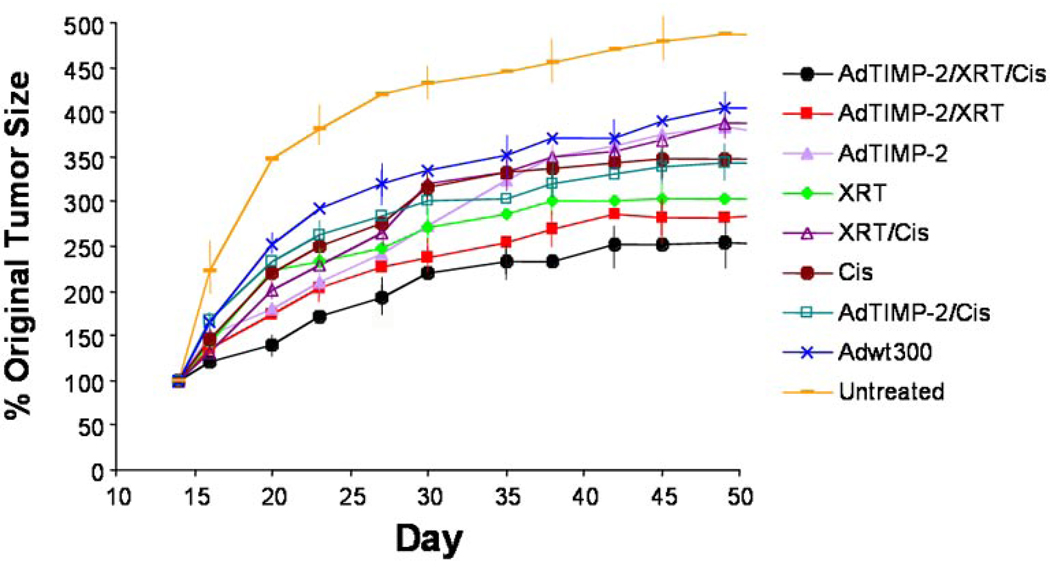
Figure 7. Therapy with Ad-TIMP-2, radiation, and Cisplatin of SCC-1 tumor xenografts.
Nude mice were inoculated with SCC-1 tumor tissue s.c. in the flank. Treatment started once the tumors were 6–8 mm in diameter. Mice were randomly divided into groups of 7 mice to receive different treatments: (1) Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin; (2) Ad-TIMP-2 + Ad Wt 300 + XRT; (3) Ad-TIMP-2 + Ad Wt 300; (4) XRT; (5) XRT + Cisplatin; (6) Cisplatin; (7) Ad-TIMP-2 + Ad Wt 300 + Cisplatin; (8) Ad Wt 300; and (9) untreated control. Animals injected with virus received 1 × 108 particles in 50 µl of Ad-TIMP-2 and Ad Wt 300 with a microsyringe for three separate needle passes. Animals receiving virus were injected intratumorally on days 2 and 8 after tumor implantation. Animals in the radiation group received 8 Gy of 60Co radiation to the tumor mass divided into four 2 Gy fractions on days 0, 4, 8, and 12. Animals received 4 i.p. doses of Cisplatin (3 mg/kg) 1 h before each fraction of radiation. The triple treatment group (Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin) had the highest growth delay followed by the two viruses + XRT group which fared better than XRT alone, Cisplatin alone, virus alone, and untreated control groups. The triple treatment group was significantly different than the control group (P=0.001). Error bars were included on alternate data points.
RESULTS
ANALYSIS OF TIMP-2 EXPRESSION
To investigate the level of TIMP-2 expression after adenoviral transduction of tumor cells, supernatants and cell lysates were collected from SCC1 cells infected with Ad-TIMP-2 + Ad Wt 300 or Ad-TIMP-2 alone. TIMP-2 levels were determined by Western blot (Fig. 1). The SCC1 cells were infected with a minimal quantity of non-replicative Ad-TIMP-2 or non-replicative Ad-TIMP-2 + Ad Wt 300 to determine if the combination would result in an increased production of TIMP-2 protein. The non-replicative Ad-TIMP-2 supernatant and cell lysate did not show higher levels of TIMP-2 than the PBS control as expected with 1 MOI of non-replicative Ad-TIMP-2. Significantly greater levels of TIMP-2 protein were expressed with the Ad-TIMP-2 + Ad Wt 300 (replicative Ad-TIMP-2) combination as compared to replication deficient Ad-TIMP-2 alone or uninfected controls.
CELL VIABILITY
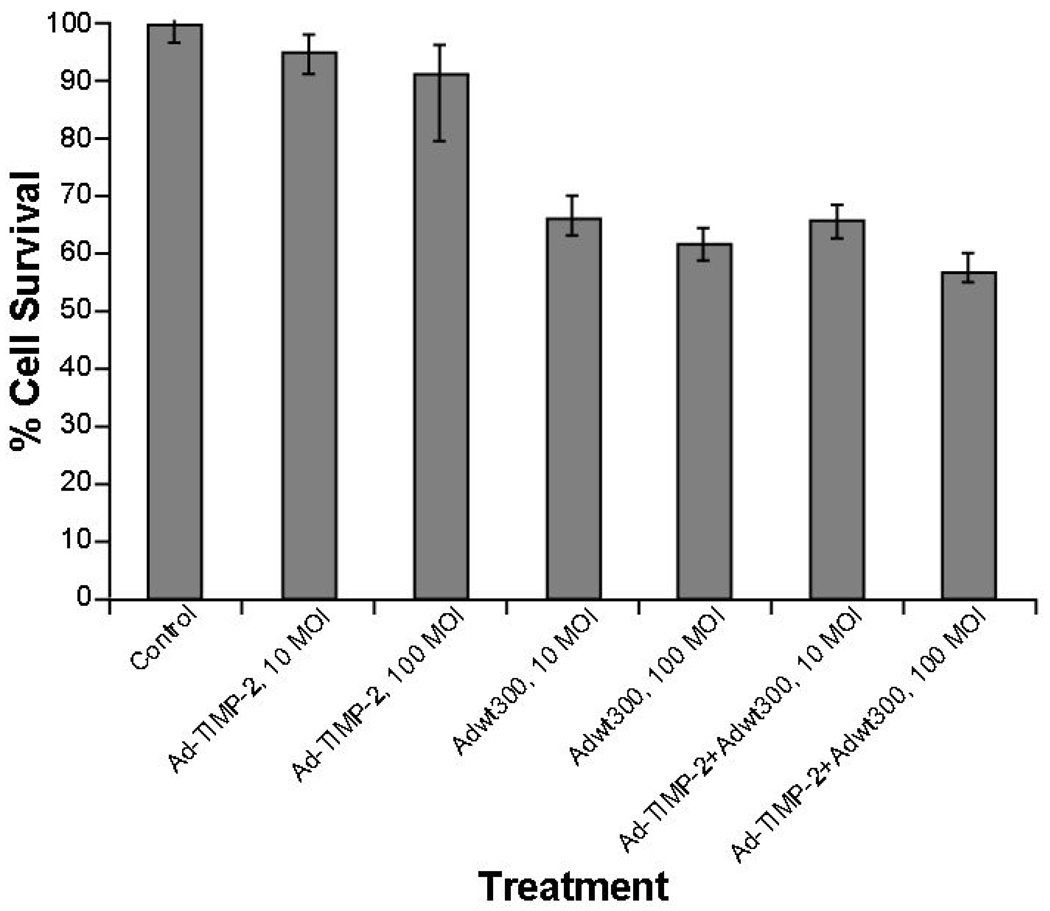
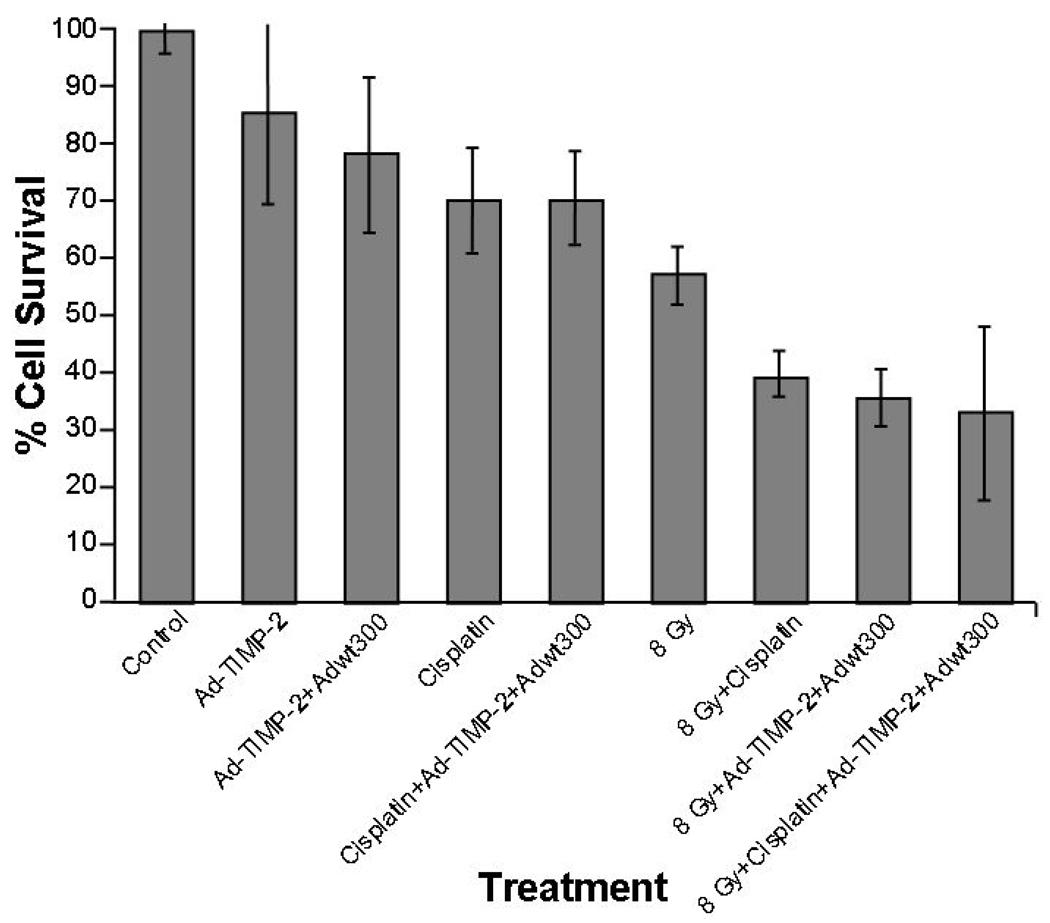
The effects of Ad-TIMP-2 (with and without Ad Wt 300), radiation, Cisplatin, and various combinations were evaluated through cell viability on SCC1 cells. No significant differences were observed in cell viability with Ad-TIMP-2 alone at 10 and 100 MOI in comparison to untreated cells. Upon addition of Ad Wt 300 + Ad-TIMP-2 cell viability was reduced by 35% after 48 h at (Fig. 2A). Treatment with the Ad-TIMP-2, radiation (8 Gy), or Cisplatin alone resulted in a moderate reduction in cell viability (23–30%) after 48 h. Combination treatments with replicative Ad-TIMP-2, Cisplatin and radiation, 8 Gy, resulted in the greatest cell viability decrease at 48 h (67%) (Fig. 2B). Treatment with Ad-TIMP-2, Ad-TIMP-2 + Ad Wt 300, Cisplatin, or combination of Cisplatin + Ad-TIMP-2 + Ad Wt 300 resulted in a significant reduction in cell viability when compared to no treatment. Radiation alone had a significant cell killing effect (p=0.011). The combination of radiation with Cisplatin increased the cell killing when compared to radiation alone (p=0.025). The triple combination of radiation, Cisplatin, and either Ad-TIMP-2 or Ad-TIMP-2 + Ad Wt 300 produced the greatest amount of cell kill compared to no treatment (p=0.001).
Figure 2. Inhibition of proliferation by Ad-TIMP-2, radiation, or Cisplatin.
(A) SCC1 cells were plated in duplicate in 96-well plates. Cells were treated with virus (Ad-TIMP-2 or Ad-TIMP-2 + Ad Wt 300) at 10 or 100 MOI. Cell viability was decreased by 35–45% at 48 h after infection with Ad-TIMP-2 and Ad Wt 300 compared to untreated control cells. (B) SCC1 cells were plated in duplicate in 96-well plates. Cells were treated with Ad-TIMP-2, Ad-TIMP-2 + Ad Wt 300, Cisplatin, or radiation alone or in combination. Treatment with the Ad-TIMP-2, radiation (8 Gy), or Cisplatin alone resulted in 23–30% reduction in cell viability after 48 h. Combination treatment with replicative Ad-TIMP-2, Cisplatin and radiation produced a 67% decrease in cell viability at 48 h. The combination of radiation with Cisplatin increased the cell killing when compared to radiation alone (p=0.025). The triple combination of radiation, cisplatin, and either Ad-TIMP-2 or Ad-TIMP-2 + Ad Wt 300 was the most significant (p=0.001).
COLLAGEN DEGRADATION ASSAY
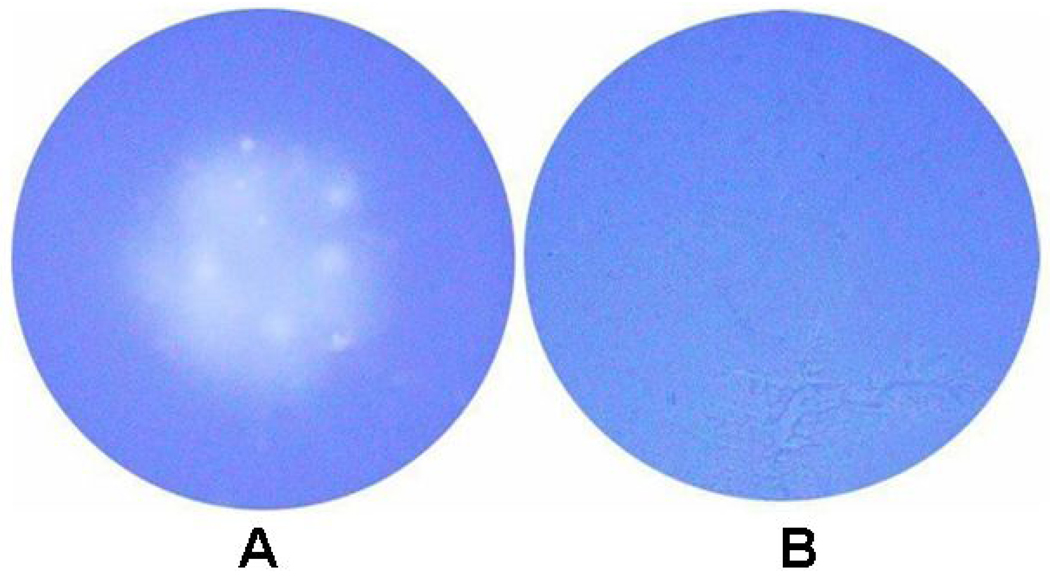
In order to determine if Ad-TIMP-2 infected cells produced a functional TIMP-2 protein, SCC-1 cells were infected with replication defective Ad-TIMP-2 virus or uninfected. Conditioned medium was filtered and added to collagen coated plates with new SCC-1 cells. Collagenolysis was observed in untreated cells when compared to cells treated with Ad-TIMP-2 conditioned medium (Fig. 3). These results indicated the anti-MMP activity of TIMP-2 produced by the replication defective Ad-TIMP-2 vector.
Figure 3. Inhibition of collagen degradation with TIMP-2.
SCC1 cells were plated in 6-well plates and incubated overnight at 37°C. To produce conditioned media, cells were (A) untreated or (B) treated for 24 h with Ad-TIMP-2 virus. After 48 h, conditioned media was removed from each set of cells and filtered with a 100,000 MW filter to remove infective virus. Untreated conditioned media (A) and Ad-TIMP-2 conditioned media (B) were added to new SCC-1 cells on collagen-coated wells for 4 days. Wells were washed and stained with Coomassie blue (0.2%), and the extent of collagenolysis in the center of the well was determined.
EFFECT OF AD-TIMP-2 WITH CHEMORADIATION ON CELLULAR APOPTOSIS
To determine the mechanism by which decreased cell viability occurs with combination therapy, SCC1 cells were infected with Ad-TIMP-2 + Ad Wt 300, Ad-TIMP-2 alone, or Ad Wt 300 alone. Cells were treated with 0 Gy, 4 Gy, and 8 Gy prior to treatment with virus. The cells were fixed and subjected to Annexin-V FITC and propidium iodide (Table 1). No apoptotic cells were seen in the fluorescein-negative control. SCC1 cells treated with radiation at 4 Gy and 8 Gy demonstrated only occasional apoptotic cells, which was not significantly different when compared with untreated control cells (p>0.05).
IMMUNOHISTOCHEMISTRY OF AD HEXON AND ANGIOGENESIS IN VIVO
Tumor xenografts were treated with i.t. injected Ad-TIMP-2 + Ad Wt 300 or untreated. After 2 weeks, tumors were removed for immunohistochemistry staining of Ad hexon protein. Hexon protein was detectable in all samples injected with virus of (Fig. 4 A and B) and undetectable in all control samples (Fig. 4C). The distribution of the positive cells within the tumor samples was diffuse; but many tumors showed focal areas of positive staining. However, the amount of infected tumor cells differed significantly among the xenografts.
To investigate the effect replicative Ad-TIMP-2 gene therapy and in combination treatments on tumor growth, was injected into preestablished human SCC1 squamous cell carcinoma tumors grown in athymic mice. A second injection was performed 7 days after the first injection, and tumor growth was monitored for 14 days after the beginning of the treatment. To assess the effect of replicative Ad-TIMP-2 with chemoradiation on tumor angiogenesis, we performed immunohistochemistry staining with Factor VIII (Fig. 5). As shown in Fig. 5, there was a sustained and significant arrest of blood vessel growth in tumors treated with Ad-TIMP-2, XRT, and Cisplatin compared with no treatment, Ad-TIMP-2 alone, XRT alone, and XRT + Cisplatin, at day 22 after the initiation of treatment. Similar results were observed in tumors at day 30.
Anti-angiogenic effects included a reduction of blood vessel size or number (P=0.028) when tumors were treated with replicative Ad-TIMP-2, but the most significant reduction in blood vessels occurred in the triple treated group when compared to untreated control (P=0.002) (Fig. 6). Although combination treatments of replicative Ad-TIMP-2 + XRT, replicative Ad-TIMP-2 + Cisplatin, and replicative Ad-TIMP-2 alone resulted in moderate anti-angiogenic response when compared to no treatment , combination treatment with replicative Ad-TIMP-2 + XRT + Cisplatin (P=0.002) resulted in superlative anti-angiogenic effects. No significant antiangiogenic effects were observed with XRT, Cisplatin, or XRT + Cisplatin, without replicative Ad-TIMP-2 (P>0.05) (Fig. 6). The co-administration of replicative Ad-TIMP-2, XRT, and Cisplatin resulted in a synergistic effect on angiogenesis (Fig. 5 and Fig. 6). Not only were there significantly fewer blood vessels in the triple treatment group, the existing blood vessels were smaller. This observation correlated well with the growth inhibition in vivo (Fig. 7). Indeed, the combination treatment with radiation + Ad-TIMP-2 led to marked inhibition of intratumor and peritumor neovascularization. These results indicate that replicative Ad-TIMP-2 gene therapy with chemoradiation effectively inhibited angiogenesis and tumor growth in vivo.
EFFECT OF VIRUS, RADIATION, AND CISPLATIN TREATMENT ON TUMOR GROWTH
Administration of Ad-TIMP-2 + Ad Wt 300, radiation, and Cisplatin markedly improved the mean growth delay of SCC1 tumors (Fig. 7). The triple treatment group (Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin) had the highest growth delay followed by the virus + ionizing radiation group which fared better than the ionizing radiation alone, Cisplatin alone, virus alone, and control groups. Tumor volume doubling time, the single treatment groups (Ad Wt 300 alone, Ad-TIMP-2 + Ad Wt 300 alone, radiation alone, and Cisplatin alone) showed little initial difference compared with the control group (20, 23, 20, 20, and 16 days, respectively). In contrast, combining Ad-TIMP-2 with ionizing radiation significantly increased the anti-tumor effect of ionizing radiation and the combination of Ad-TIMP-2, radiation, and Cisplatin increased the anti-tumor effect even more (29 days). The control group had tumor doubling by day 16 (median) compared to day 29 for the virus, radiation, and Cisplatin group. Tumor doubling in the Ad-TIMP-2 + Ad Wt 300, radiation, and Cisplatin treated group was significantly different than the control group (P=0.001) and the radiation group. Treatment with any single agent alone or radiation + Cisplatin produced limited tumor growth inhibition compared to the control group. The results also remained significant over time when combined treatment (Ad-TIMP-2 + Ad Wt 300 + XRT + Cisplatin) was compared with ionizing radiation alone at day 70 (P=0.01).
DISCUSSION
Conventional radiation therapy and chemotherapy are cytotoxic, but affect normal cells as well as cancer cells. Since a therapeutic dose must be reduced to limit host toxicity, cancer cells often survive and eventually become resistant. To address this problem, targeted therapies have been successfully combined with cytotoxic treatment improving response without increased toxicity [27]. Following this reasoning, we tested the hypothesis that the combination of a TIMP-2 overexpressing Ad vector with conventional therapies would augment the efficacy of the treatment in vivo. The combination of intratumoral injection of Ad-TIMP-2 + Ad Wt 300, Cisplatin, and radiation therapy is more effective in suppressing the growth of subcutaneous SCC1 xenografts in nude mice than injection of virus alone, Cisplatin alone, or radiation treatment alone (Fig. 7). A significant increase in anti-tumor and anti-angiogenic effect was achieved by combining the three modalities (Fig. 5 and Fig. 7).
The recognition that MMPs moderate the process of tumor growth and metastasis and the subsequent development of a large number of agents able to inhibit the MMP activity has led to the evaluation of several synthetic MMP inhibitors in early clinical trials and randomized clinical trials for which the first results are now available. The initial enthusiasm on the possible use of MMP inhibitors in the treatment of cancer has clearly been dampened because of a failure to show efficacy. However, it is now known that cytostatic agents may work better in combination with conventional cytotoxic treatment. To that end, we accessed the potential of TIMP-2 in combination with radiation and Cisplatin treatment in HNSCC xenografts.
Adenovirus has been employed for human gene therapy applications in which their safety profile has been established to deliver both cytotoxic and cytostatic agents for the treatment of cancer including advanced HNSCC [2]. One advantage of gene therapy using TIMPs is that constitutative expression of a naturally occurring compound can be introduced to a local area to prevent further tumor growth and metastasis. Additionally, as TIMP-2 is not cytotoxic to the cell, viral replication may occur in the infected cells to produce more TIMP-2 without resulting in viral attenuation. Recently, adenovirus has been armed with anti-angiogenic genes and when used in combination with chemotherapy has resulted in increased tumor response [28].
It has been reported that a replication-deficient therapeutic adenovirus can coamplify with replicative adenoviruses [24]. The resulting selective production of large numbers of therapeutic adenovirus particles in situ within a tumor mass could transduce neighboring tumor cells to increase overall transduction efficiency, and result in an increase in the amount of the therapeutic protein [24]. Indeed, we found increased TIMP-2 production with the co-administration of Ad-TIMP-2 + Ad Wt 300 at low MOI when compared to replication-deficient Ad-TIMP-2 alone in cells (Fig. 1). Non-replicative Ad TIMP-2 alone did not result in increased TIMP-2 levels when compared to uninfected cells at low MOI as expected (Fig 1). Since SCC1 cells in vitro treated with non-replicative Ad-TIMP-2 alone did not result in decreased cell viability and replicative Ad-TIMP-2 resulted in a 35% decrease (Fig. 2A), the cytotoxicity was attributed to the lytic cycle of the virus alone instead of the TIMP-2 protein since TIMP-2 is cytostatic and not cytotoxic [15]. TIMP-2 has anti-tumor activity, such as inhibiting MMPs and angiogenesis, within the in vivo microenvironment which may not be apparent in vitro[20, 29]. The growth of SCC1 cells in vitro was inhibited by high dose radiation, 8 Gy, but not by a lower dose of radiation, 4 Gy after 24 h (data not shown). Combination of 8 Gy radiation and replicative virus resulted in an additive decrease in SCC1 cell viability of 67% after 48 h. The combination of Cisplatin, 8 Gy, and replicative Ad-TIMP-2 produced a decrease in cell viability equal to 67% at 48 h. Although SCC1 cells did not appear to undergo significant levels of apoptosis after treatment with radiation and virus, a high number of cells stained with propidium iodide indicating cell death (Table 1).
Although the replicative Ad-TIMP-2 did not affect tumor cell viability in vitro when compared to Ad Wt 300 alone (Fig. 2A), it was efficacious against tumor growth in vivo (Fig. 7). The limited effect of TIMP-2 in vitro is not surprising as similar results have been reported previously [20]. TIMP-2 is an inhibitor of FGF-stimulated endothelial cell and capillary endothelial cell proliferation, as well as a MMP inhibitor [29]. The anti-angiogenic approach targets the tumor endothelium instead of the tumor, which can amplify the tumor killing effect. As anti-angiogenic gene therapy is a cytostatic approach, its efficiency depends largely on a sustained secretion of transgene products at a high level by virally-infected cells. The use of replicative Ad-TIMP-2 alone or in combination treatment resulted in a significant antiangiogenic response (Fig. 6), and multimodality treatment with replicative Ad-TIMP-2, XRT, and Cisplatin resulted in a synergistic anti-angiogenic and anti-tumor response (Fig. 5 and Fig. 7).
To confirm successful administration of virus into the tumors, immunohistochemistry for hexon protein was preformed. Adenovirus hexon staining revealed needle tracts (Fig. 4A) and additional distant foci of positive cells (Fig. 4B) 2 days subsequent to the final injection of Ad-TIMP-2 + Ad Wt 300 thus confirming that the virus could spread throughout the xenograft tumor.
The use of a replicative adenoviral vector produces increased expression of a transgene over time resulting in a more sustained therapeutic effect and reduces the initial dose of vector required and potential toxicity. The inhibitory effect on tumor growth was associated with a markedly decreased vascularization within the tumors (Fig. 5). The anti-angiogenic approach targets the tumor endothelium instead of the tumor, which can amplify the tumor killing effect. Treatment with replicative Ad-TIMP-2 alone resulted in smaller blood vessels within tumor xenografts when compared to untreated control tumors (Fig. 5C and Fig. 5H). Additionally, combination treatment with either XRT or Cisplatin and replicative Ad-TIMP-2, or replicative Ad-TIMP-2 alone, resulted in similar anti-tumor responses and an overall reduction in abundance of blood vessels when compared to untreated control tumors (Fig. 5B, 5C, 5G, and Fig. 7). The triple combination treatment in vivo resulted in significantly fewer and smaller blood vessels (Fig. 5A). The anti-angiogenic effect of TIMP-2 in combination treatments may prove to be as important as the MMP inhibition effect. TIMP-2, by inhibiting MMP activity, may be a good choice for cancer therapy since it simultaneously targets invasive tumor cells and tumor angiogenesis [20].
Although the TIMP-2 did not affect tumor cell viability in vitro (Fig. 2A), it could be efficacious against tumor growth in vivo (Fig. 7). We found that multimodality treatment of in vivo HNSCC with Ad-TIMP-2 gene therapy and chemoradiation therapy resulted in significant anti-tumor effect and improved survival of mice (Fig. 7). In mouse xenografts, we found that chemotherapy combined with armed adenovirus, radiation combined with armed adenovirus, and chemoradiation therapy combined with armed adenovirus resulted in a significantly greater response over single-agent therapies.
Interestingly, the combination of Ad-TIMP-2 + XRT + Cisplatin inhibited tumor growth and angiogenesis as well as resulted in less mortality. This clearly indicates that the efficacy and potency of this gene therapy in a mouse xenograft tumor model were greatly improved by the association of TIMP-2 with chemoradiation. The tumor regression achieved by the combined treatment was most likely attributable to the complementary actions of TIMP-2 and XRT/Cisplatin, as TIMP-2 mainly inhibits both endothelial and cancer cell migration and radiation and Cisplatin are cytotoxic. To our knowledge, this is the first time that the beneficial action gene therapy, radiation, and chemotherapy in combination has been shown in vivo in head and neck squamous cell carcinoma.
In conclusion, the combination of TIMP-2-overexpressing Ad virus, chemotherapy, and radiation as anti-cancer therapy and demonstrated significant anti-tumor activity and antiangiogenesis allowed for a much lower effective dose of the three agents than would have been necessary had we used a single modality. TIMP-2 gene therapy enhanced the therapeutic effects of viral therapy in HNSCC as both an MMP inhibitor and an anti-angiogenic agent, which was further enhanced by chemoradiation therapy. This, translated onto a clinical setting, should mean much less side effects and morbidity in the patient.
Acknowledgements
The authors thank C. Hardy and J. Sellers for expert technical assistance and S. Lagan for manuscript preparation. This work was supported by the NIH Training Grant T32 CA075930.
REFERENCES
- 1.Lemaire F, Millon R, Young J, Cromer A, Wasylyk C, Schultz I, Muller D, Marchal P, Zhao C, Melle D, Bracco L, Abecassis J, Wasylyk B. Differential expression profiling of head and neck squamous cell carcinoma (HNSCC) Br J Cancer. 2003;89:1940–1949. doi: 10.1038/sj.bjc.6601373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee JG, Li D, O'Malley BW, Jr, Suntharalingam M. Combination radiation and adenovirus-mediated P16(INK4A) gene therapy in a murine model for head and neck cancer. ORL J. Otorhinolaryngol. Relat. Spec. 2003;65:144–154. doi: 10.1159/000072252. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigo JP, Suarez C, Ferlito A, Devaney KO, Petruzzelli GJ, Rinaldo A. Potential molecular prognostic markers for lymph node metastasis in head and neck squamous cell carcinoma. Acta Otolaryngol. 2003;123:100–105. doi: 10.1080/0036554021000028073. [DOI] [PubMed] [Google Scholar]
- 4.Morley S, MacDonald G, Kirn D, Kaye S, Brown R, Soutar D. The dl1520 virus is found preferentially in tumor tissue after direct intratumoral injection in oral carcinoma. Clin. Cancer Res. 2004;10:4357–4362. doi: 10.1158/1078-0432.CCR-03-0443. [DOI] [PubMed] [Google Scholar]
- 5.Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, Landers S, Maples P, Romel L, Randlev B, Reid T, Kaye S, Kirn D. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 6.Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 7.Gress TM, Muller-Pillasch F, Lerch MM, Friess H, Buchler M, Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int. J. Cancer. 1995;62:407–413. doi: 10.1002/ijc.2910620409. [DOI] [PubMed] [Google Scholar]
- 8.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 9.Huppertz B, Kertschanska S, Demir AY, Frank HG, Kaufmann P. Immunohistochemistry of matrix metalloproteinases (MMP), their substrates, and their inhibitors (TIMP) during trophoblast invasion in the human placenta. Cell Tissue Res. 1998;291:133–148. doi: 10.1007/s004410050987. [DOI] [PubMed] [Google Scholar]
- 10.Patterson ML, Atkinson SJ, Knauper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503:158–162. doi: 10.1016/s0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 12.Ramnath N, Creaven PJ. Matrix metalloproteinase inhibitors. Curr. Oncol. Rep. 2004;6:96–102. doi: 10.1007/s11912-004-0020-7. [DOI] [PubMed] [Google Scholar]
- 13.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorogh T, Beier UH, Baumken J, Meyer JE, Hoffmann M, Gottschlich S, Maune S. Metalloproteinases and their inhibitors: influence on tumor invasiveness and metastasis formation in head and neck squamous cell carcinomas. Head Neck. 2006;28:31–39. doi: 10.1002/hed.20298. [DOI] [PubMed] [Google Scholar]
- 15.Imren S, Kohn DB, Shimada H, Blavier L, DeClerck YA. Overexpression of tissue inhibitor of metalloproteinases-2 retroviral-mediated gene transfer in vivo inhibits tumor growth and invasion. Cancer Res. 1996;56 2891289-2891285. [PubMed] [Google Scholar]
- 16.Moses MA. The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells. 1997;15:180–189. doi: 10.1002/stem.150180. [DOI] [PubMed] [Google Scholar]
- 17.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMPindependent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 18.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 19.Indraccolo S, Minuzzo S, Gola E, Habeler W, Carrozzino F, Noonan D, Albini A, Santi L, Amadori A, Chieco-Bianchi L. Generation of expression plasmids for angiostatin, endostatin and TIMP-2 for cancer gene therapy. Int J Biol Markers. 1999;14:251–256. doi: 10.1177/172460089901400410. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Lindenmeyer F, Grenet C, Opolon P, Menashi S, Soria C, Yeh P, Perricaudet M, Lu H. AdTIMP-2 inhibits tumor growth, angiogenesis, and metastasis, and prolongs survival in mice. Hum. Gene Ther. 2001;12:515–526. doi: 10.1089/104303401300042429. [DOI] [PubMed] [Google Scholar]
- 21.Gately S, Kerbel R. Antiangiogenic scheduling of lower dose cancer chemotherapy. Cancer J. 2001;7:427–436. [PubMed] [Google Scholar]
- 22.Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, Robertson AG, Park O, Gulley ML, Heise C, Von Hoff DD, Kaye SB. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 23.Baker AH, Wilkinson GW, Hembry RM, Murphy G, Newby AC. Development of recombinant adenoviruses that drive high level expression of the human metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 and -2 genes: characterization of their infection into rabbit smooth muscle cells and human MCF-7 adenocarcinoma cells. Matrix Biol. 1996;15:383–395. doi: 10.1016/s0945-053x(96)90158-4. [DOI] [PubMed] [Google Scholar]
- 24.Lee CT, Park KH, Yanagisawa K, Adachi Y, Ohm JE, Nadaf S, Dikov MM, Curiel DT, Carbone DP. Combination therapy with conditionally replicating adenovirus and replication defective adenovirus. Cancer Res. 2004;64:6660–6665. doi: 10.1158/0008-5472.CAN-04-1200. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal EL, Zhang W, Talbert M, Raisch KP, Peters GE. Extracellular matrix metalloprotease inducer-expressing head and neck squamous cell carcinoma cells promote fibroblast-mediated type I collagen degradation in vitro. Mol. Cancer Res. 2005;3:195–202. doi: 10.1158/1541-7786.MCR-04-0203. [DOI] [PubMed] [Google Scholar]
- 26.Aznavoorian S, Moore BA, Alexander-Lister LD, Hallit SL, Windsor LJ, Engler JA. Membrane type I-matrix metalloproteinase-mediated degradation of type I collagen by oral squamous cell carcinoma cells. Cancer Res. 2001;61:6264–6275. [PubMed] [Google Scholar]
- 27.Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N, Lee J, Pennathur-Das R, Radzyminski J, Wypych J, Brignetti D, Scott S, Stephens J, Karpf DB, Henderson DR, Yu DC. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res. 2001;61:5453–5460. [PubMed] [Google Scholar]
- 28.Zhang Z, Zou W, Wang J, Gu J, Dang Y, Li B, Zhao L, Qian C, Qian Q, Liu X. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol. Ther. 2005;11:553–562. doi: 10.1016/j.ymthe.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J. Cell Physiol. 1993;157:351–358. doi: 10.1002/jcp.1041570219. [DOI] [PubMed] [Google Scholar]