Figure 2.
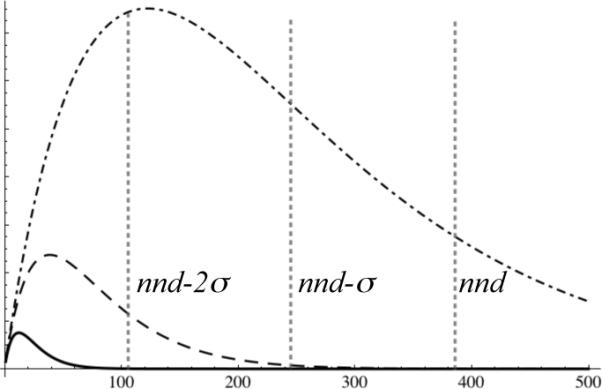
The relative number of different potential reaction events, , among solute molecules that may be encountered by hydroxyl radicals as a function of distance (Å) from a point source, in 10% v/v glycerol (solid curve at lower left corner), 1% v/v glycerol (dashed curve), and 0.1% v/v glycerol (dot-dashed curve). The vertical scale is arbitrary. Glycerol is considered part of the solvent. Also shown, for a 5 μM protein solution, are vertical lines marking the average protein-protein nearest-neighbor distance nnd = 384Å, and distances one standard deviation (139Å) closer, nnd – σ, and two standard deviations closer, nnd – 2σ. For a 5 μM solution containing 10% v/v glycerol, hydroxyl radical encounters are effectively confined to the protein complex from which the radical originates.
