FIGURE 5.
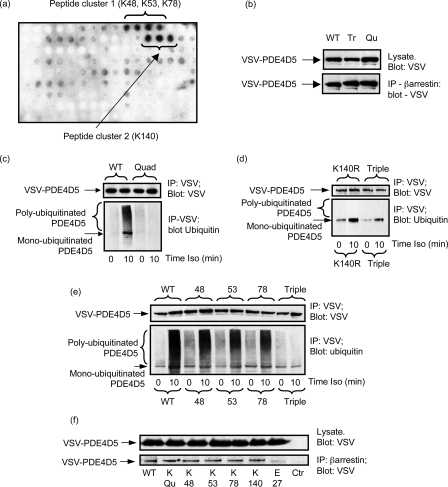
PDE4D5 ubiquitination sites. a, a scanning peptide array of the entire 745-amino acid sequence of PDE4D5 was generated using overlapping immobilized 25-mer peptides each displaced by 5 residues. This was subjected to in vitro ubiquitination with recombinant Mdm2 and subsequent detection of bound ubiquitin using an anti-ubiquitin antibody. The dark spots indicate positive interacting peptides, whose sequence incorporated the indicated lysine residues. b, HEKB2 cells were transfected with either wild-type VSV-PDE4D5 or the Quad (K48R:K53R:K78R:K140R)-VSV-PDE4D5 or Triple (K48R:K53R:K78R)-VSV-PDE4D5 mutant forms. Lysates expressing equal amounts of each VSV-PDE4D5 species were taken, then β-arrestin immunopurified, and the amount of associated VSV-PDE4D5 species was identified by immunoblotting. Quantitative densitometry showed no difference (<10%) between the amounts of these species sequestered by β-arrestin in resting cells. c–e, HEKB2 cells were transfected to express either wild-type or mutant forms of VSV-tagged PDE4D5 and, where indicated, challenged for 10 min with isoprenaline (10 μm). VSV-PDE4D5 was immunopurified and blotted for VSV and ubiquitin. In c Quad/Qu mutant is (K48R:K53R:K78R:K140R), in d Triple/Tr is (K48R:K53R:K78R) and K140R is Lys-140 → Arg, and in e, 48 is K48R, 53 is K53R, and 78 is K78R. f, HEKB2 cells were transfected with the arginine mutants of indicated residues in VSV-tagged PDE4D5, endogenous β-arrestin was immunoprecipitated using a specific antiserum and the immunoprecipitates blotted with a VSV-specific antiserum to detect PDE4D5. Quad indicates the (K48R:K53R:K78R:K140R)-VSV-PDE4D5 species and Ctr is the beads only control where no β-arrestin was used in the immunoprecipitation (IP) protocol. Data show experiments typical of ones performed three times.