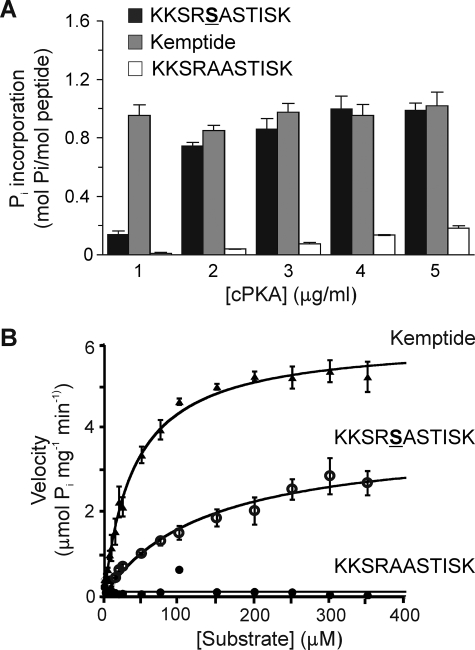
FIGURE 8.
Stoichiometric and kinetic analysis of PKA-catalyzed 32P incorporation by synthetic peptide. Synthetic peptide KKSRSASTISK, corresponding to amino acid residues 445–455 of Kv1.2 and containing the putative PKA site Ser-449, was compared as a substrate to LLRRASLG (Kemptide) (positive control) and KKSRAASTISK (corresponding to the sequence of Kv1.2-S449A mutant, negative control). A, fixed concentrations of each peptide (50 μm) were treated with [γ-32P]ATP and cPKA (1.0–5.0 μg/ml), and 32P incorporation was quantified by C̆erenkov counting. Data are the means ± S.E. of three independent experiments carried out in triplicate. B, cPKA activity was determined by quantification of 32P incorporation into KKSRSASTISK, Kemptide, and KKSRAASTISK peptides during the linear phase of the reaction (t = 1.5 min). Enzyme velocities were plotted against substrate concentration and fitted to the Michaelis-Menten equation to obtain Km and Vmax values.