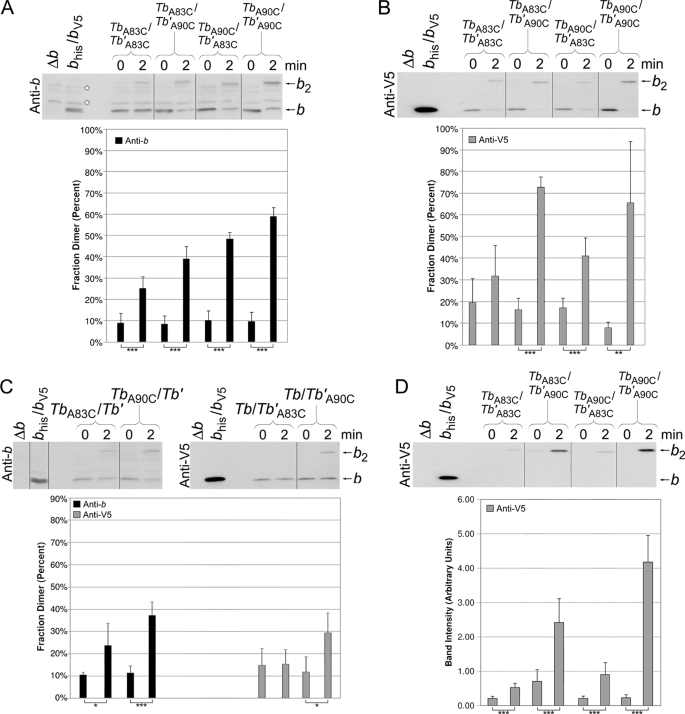
FIGURE 4.
Disulfide cross-link formation in F1F0 ATP synthase peripheral stalks composed of chimeric Tb and Tb′ subunits. The membranes were prepared from strains coexpressing cysteine-containing Tb and Tb′ subunits. Samples from KM2/pSBC123/pSBC125 (TbA83C/Tb′A83C), KM2/pSBC123/pSBC126 (TbA83C/Tb′A90C), KM2/pSBC124/pSBC125 (TbA90C/Tb′A83C), and KM2/pSBC124/pSBC126 (TbA90C/Tb′A90C) were cross-linked with 500 μm Cu2+ exactly as described for Fig. 2 and analyzed by Western blot using antibodies against the b subunit (A) and V5 epitope tag (B) (10 and 1 μg of protein/lane, respectively). The vertical lines indicate the removal of intervening lanes in silico. Samples with a low probability of being identical are indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001; n = 5). C, membranes were prepared from strains coexpressing a cysteine-containing chimeric subunit with the complementary cysteine-free chimeric subunit. Samples from KM2/pSBC123/pSBC98 (TbA83C/Tb′), KM2/pSBC124/pSBC98 (TbA90C/Tb′), KM2/pSBC97/pSBC125 (Tb/Tb′A83C), and KM2/pSBC97/pSBC126 (Tb/Tb′A90C) were cross-linked as before and analyzed by Western blot. Antibodies against either the b subunit or the V5 epitope tag were used for the detection of (Tb)2 and (Tb′)2 dimers, respectively. The experiment was done a total of four times, and the average fraction dimer is graphed below each lane. D, disulfide cross-link formation in heterodimeric Tb/Tb′ peripheral stalks was detected using a Nickel-resin purification assay as previously described (22). Samples KM2/pSBC123/pSBC125 (TbA83C/Tb′A83C), KM2/pSBC123/pSBC126 (TbA83C/Tb′A90C), KM2/pSBC124/pSBC125 (TbA90C/Tb′A83C), and KM2/pSBC124/pSBC126 (TbA90C/Tb′A90C) were prepared and cross-linked as before. Each sample was then solubilized and purified over a high capacity nickel chelate affinity matrix to retain only F1F0 complexes containing at least one histidine tag. A total of 10% of the protein retained by the high capacity nickel chelate affinity matrix resin was analyzed by Western blot using an antibody against the V5 epitope tag. The average fraction dimer is graphed below each lane (n = 8).