Abstract
Alterations in central nervous system response to menstrual cycle-related fluctuations in neuroactive steroids are thought to underlie the emergence of negative affect in the luteal phase of the menstrual cycle in women with premenstrual dysphoric disorder (PMDD). Such changes in the neuroendocrine milieu may lead to heightened arousal and response to stress in women with PMDD. Using the acoustic startle paradigm, we sought to determine whether women with PMDD have an accentuated physiologic response to a mildly aversive stimulus during the luteal compared to follicular phase. Further, we also examined the impact of visual affective stimuli on acoustic startle response (ASR) magnitude. During the follicular and luteal phases of the menstrual cycle, acoustic stimuli (103 dB) were delivered to 15 women with PMDD and 14 healthy menstruating women of similar age. After obtaining baseline ASR, the procedure was repeated when subjects viewed pleasant, neutral and unpleasant pictures. There was a significant group by menstrual cycle phase interaction for baseline ASR magnitude, which can be attributed to the heightened startle magnitude in women with PMDD compared to healthy women during the luteal relative to the follicular phase. The direction and degree to which picture viewing modulated the startle magnitude did not vary by group or menstrual cycle phase. These data suggest that menstrual cycle phase has a powerful modulatory effect on physiologic reactivity in women with PMDD but not in healthy women. Physiologic response to affective stimuli appears to be intact in women with PMDD across the menstrual cycle.
Keywords: PMDD, startle, menstrual cycle, affect, valence, arousal
INTRODUCTION
It is generally held that disruptions in the normal interplay between neuroactive steroids and their brain targets underlie the pathogenesis of negative affective states associated with periods of hormonal fluctuation such as the premenstruum, puerperium and perimenopause (Epperson et al, 1999; Bernardi et al, 2004; Eriksson et al, 2006; N-Wihlback et al, 2006; Sundstrom-Poromaa et al, 2003). Although the majority of women experience some mood and/or physical symptoms before or during their period, between 3 and 8% of premenopausal women experience the more severe mood disorder known as premenstrual dysphoric disorder (PMDD). PMDD is characterized by a constellation of mood and physical symptoms, which are present during the week before the onset of menses, absent in the postmenstrual week, and hamper functioning in at least one domain of life. Individuals are required to have five or more symptoms with this temporal pattern that are at least moderate in severity. One of the five symptoms must be sadness/depressed mood, anger/irritability, anxiety/tension, or mood lability to meet DSM-IV research criteria (American Psychiatric Association, 1994).
Negative affect/distress is menstrual cycle phase-dependent in women with PMDD and allows for the within-subject examination of physiologic measures under two disparate psychological and hormonal states. Neuroactive steroids such as estradiol and progesterone as well as the progesterone metabolite and GABAA receptor agonist 3α, 5α tetrahydroprogesterone (allopregnanolone; ALLO) have pronounced effects on brain chemistry and function. They modulate those neurotransmitters and brain regions involved in the organism’s behavioral response to a brief, mildly noxious auditory stimulus otherwise known as the startle reflex (Davis and Gilmore, 1998; Koch, 1999; Van den Buuse and Eikelis, 2001; He et al, 2005; Van Nobelen and Kokkinidis, 2006). The startle reflex is well conserved across species, is thought to be mediated by similar neural pathways regardless of species (Koch, 1999), and is sensitive to pharmacologic and hormone modulation. Thus, we sought to determine whether women with PMDD experience altered physiologic response (as measured by the ASR magnitude) to mildly noxious acoustic stimuli delivered during the luteal phase (high estradiol, high progesterone) vs the follicular phase (low estradiol, low progesterone) of the menstrual cycle.
As the ASR magnitude is highly sensitive to the emotional state of the individual (Cook et al, 1991; Bradley et al, 1993; Lang et al, 1990, 1998, 2005; Grillon and Baas, 2003), it is not only an excellent measure of physiologic arousal but is also a specific measure of how an individual processes affect at a physiologic level. In normal males and females, the ASR magnitude is accentuated and diminished by aversive and pleasurable states, respectively. As one could surmise that physiologic hyperarousal and/or negative affect may mediate a mood-related bias to the affective modulation of acoustic startle magnitude, we also examined whether women with PMDD differed from healthy controls in the direction and degree of ASR response to viewing pleasant, neutral and unpleasant pictures.
MATERIALS AND METHODS
Participants
Subjects were recruited by public advertisement and referral from area obstetricians/gynecologists. Subjects gave written informed consent for participation in this study, which was approved by the Yale University School of Medicine’s Human Investigation Committee (New Haven, CT). All subjects were healthy as determined by medical history and routine laboratory tests. Urine toxicology screens were also negative.
All subjects underwent 2 months of prospective daily ratings using the Daily Record of Severity of Problems (DRSP; Endicott and Harrison, 1990; Endicott et al, 2006) to confirm diagnosis of PMDD or status as a healthy control. Participants had regular menstrual cycles of 24–39 days duration, which were relatively consistent in length (between cycles) within subjects. Ovulation was confirmed during the screening and study months using urine ovulation test kits (ANSWER® Ovulation Tests, Church & Dwight Co., Inc., Princeton, NJ). Women using hormonal contraception within the previous 6 months or pregnant within the previous year were not eligible for participation.
The women who were included in the PMDD group met DSM-IV research criteria for PMDD (American Psychiatric Association, 1994) with the required severity and pattern of symptoms confirmed with 2 months of prospective daily mood ratings. With the first day of menstrual flow designated as day 1, women with PMDD had at least a 50% increase in five mood symptoms from the follicular phase ratings (days 5–11) to the luteal phase ratings (days −1 to −7), with at least one of the mood symptoms being irritability, mood lability, depressed mood, and/or anxiety/tension. A structured clinical interview for the DSM-IV (SCID-P; First et al, 1998) was conducted to exclude women with comorbid psychiatric or substance abuse disorders (including nicotine) within the previous 2 years. Healthy menstruating women demonstrated no evidence of premenstrual mood symptoms according to prospective daily ratings and did not meet DSM-IV criteria for a past or present Axis I disorder as assessed by the SCID DSM-III-R Non-Patient Edition (SCID-NP; Spitzer et al, 1990). As per their report, healthy women had no first-degree relatives with an Axis I psychiatric disorder, although three had a first-degree relative with a substance use/dependence disorder.
Behavioral Assessments
The DRSP (Endicott and Harrison, 1990; Endicott et al, 2006) requires women to rate their mood and physical symptoms on a scale of 1 to 6 with ‘1’ indicating that the symptom is ‘not at all’ present and ‘6’ indicating that the symptom is ‘extreme’. The final three questions included in the DRSP assess the degree to which any PMDD symptom interferes with hobbies or social life, work, and/or interpersonal relationships.
Subjects completed the state portion of the Spielberger State/Trait Anxiety Interview (STAI; Spielberger, 1984) upon arrival to the startle laboratory. The STAI is a 40-item self-rated instrument, which assesses separate dimensions of state and trait anxiety. The state anxiety portion of the STAI has 20 items rated on four-point intensity scales (1=not at all; 2=somewhat; 3=moderately; 4=very much). Ten of the items are positively worded, and 10 of them are negatively worded.
Timing of Acoustic Startle Sessions
With first day of onset of menstrual flow anchored as day 1, all subjects were scheduled for startle testing once during the early to mid-follicular phase (days 3–10) and once in the mid to late luteal phase (days −10 to −3). Follicular-phase testing was designed to capture women before the late follicular phase rise in estradiol, and thus a plasma estradiol level of ≥80 pg/ml was the ground for exclusion of data for that phase. Likewise, luteal-phase testing was scheduled to be performed when progesterone levels could be expected to have risen to above 3 ng/ml. Ovulation was estimated using a urine LH kit, but sex hormones were measured on each test day to confirm menstrual cycle phase and the above hormonal parameters. Both groups of women were balanced with respect to which menstrual cycle phase they underwent testing first.
Startle Testing
Stimulation and recording were controlled by a commercial system (Contact Precision Instruments, London, UK). The pictorial stimuli were 96 color pictures selected from the International Affective Picture System (IAPS; Lang et al, 2005). The pictures were selected across three valence categories of unpleasant, neutral, and pleasant, with each category containing 32 exemplar pictures. Examples of pleasant pictures included a romantic kiss, babies, and puppies; those of neutral pictures included a lamp, spoon and mushrooms; and the unpleasant pictures included scenes of mutilation, violent acts, and suicide. The pictures were presented on a 17-inch computer monitor positioned approximately 1m from the subjects. The blink-eliciting stimulus was a 40-ms duration, 103 dB(A) burst of white noise with a near instantaneous rise time presented binaurally through headphones. Blink reflex was measured by recording the electromyographic (EMG) activity of the orbicularis oculi muscle with two miniature Ag/AgCl electrodes filled with a standard electrolyte directly beneath the left eye approximately 1 cm apart. A ground electrode was attached to the forearm (impedance level less than 5 kΩ). Amplifier bandwidth was set to 30–500Hz.
The electrodes were attached and the recording session was started with a block consisting of nine acoustic startle stimuli delivered every 18–25 s. This initial block of acoustic stimuli, referred to as the ‘baseline’ from hereon, was followed by a session during which acoustic stimuli were administered during picture-viewing. In each session, 48 pictorial stimuli—divided into two phases of 24 pictures each (Table 1)—were presented sequentially with a 5-min break between the phases. Each phase started with the delivery of six startle stimuli. In each 24-picture phase, the pictures were presented in two blocks of four neutral, four pleasant, and four unpleasant pictures per block. Each picture was shown for 6 s. Within a block, three out of four pictures of the same valence were accompanied by an auditory blink-eliciting stimulus with picture-to-probe onset intervals of either 3.5 or 5.5 s. The study was designed so that startle stimuli were presented every 18–25 s. Four sets of 24 pictures were created and rotated across participants. Following the startle test, the pictures were shown once more one at a time and subjects were asked to rate them on dimension of valence and arousal using the 9-point Self Assessment Manikin (SAM; Bradley and Lang, 1994). In the present analysis, SAM ratings were scored so that higher ratings were associated with increased negative valence and with increased arousal.
Table 1.
Numbers of the Specific Pictures from the International Affective Picture Systema Used in This Study
| Type | Picture number |
|---|---|
| Neutral | 2221, 2440, 2840, 5534, 7000, 7004, 7006, 7009, 7010, 7020, 7025, 7030, 7031, 7035, 7040, 7050, 7060, 7080, 7090, 7100, 7130, 7150, |
| 7175, 7185, 7187, 7217, 7224, 7233, 7234, 7235, 7491, 7705 | |
| Positive | 1440, 1710, 1920, 2080, 4599, 4601, 4603, 4606, 4607, 4608, 4609, 4610, 4611, 4640, 4641, 4650, 4653, 4656, 4660, 4689, 5470, 5629, |
| 5831, 7330, 8030, 8185, 8190, 8200, 8210, 8370, 8400, 8540 | |
| Negative | 2205, 3030, 3051, 3060, 3071, 3160, 3180, 3181, 3220, 3230, 3350, 3550, 6260, 6312, 6313, 6350,6550, 6560, 6570, 9040, 9050, 9250, |
| 9400, 9405, 9410, 9433, 9800, 9810, 9910, 9911, 9920, 9921 |
Center for the Study of Emotion and Attention (CSEA-NIMH) (1999). The International Affective Picture System: Digitized Photographs. The Center for Research in Psychophysiology, University of Florida: Gainesville, FL.
Data Analysis
For the primary outcome of baseline ASR magnitude over the menstrual cycle, peak amplitude of the blink reflex was determined in the 20–120-ms time frame following stimulus onset relative to baseline (average baseline EMG level for the 50 ms immediately preceding stimulus onset). For affective modulation of the ASR magnitude, the data were averaged for each valence over blocks and phases. Initially, data were examined descriptively using means, standard deviations and graphs. Each outcome was tested for normality using Kolmogorov–Smirnov test statistics and normal probability plots. After log transformation, all data were approximately normal.
Mixed models were used to evaluate the magnitude of baseline ASR and ASR during affective modulation, and level of arousal and valence. The model for baseline ASR (log) included group (healthy females and PMDD) as a between-subject explanatory factor, and phase (follicular and luteal) as a within-subject effect. The interaction between group and phase was also modeled. For the parameter assessed during affective modulation, each outcome (log) represented the dependent variable, while stimulation (pleasant, neutral, unpleasant) was included as within-subject explanatory factor in the mixed models described above. These models allowed for testing of all two- and three-way interactions among group, phase, and stimulation. When appropriate, post-hoc comparisons were performed. In the above models, subject was used as the clustering factor and for the model assessing ASR during affective modulation; state anxiety as measured using the STAI was included as a covariate. State anxiety was analyzed as a separate outcome using similar models describing baseline ASR.
The effect of phase order for startle sessions was not significant in any of the above models and was dropped. Potential associations between plasma estradiol and progesterone and baseline ASR were explored by computing Spearman correlations. Severity of PMDD symptoms as determined by mean change in total DRSP score from the follicular phase (day 5–11) to the luteal phase (day −1 to −7) were also examined in relation to baseline ASR using Spearman correlations. All results were considered to be statistically significant at p<0.05, after adjusting for multiple tests within, but not across domains, using the Bonferroni method (Hochberg and Tamhane, 1987) as follows: baseline ASR magnitude and affective modulation of ASR magnitude adjusted for two analyses; arousal and valence adjusted for two analyses; and all correlations adjusted for four analyses. All data were analyzed using SAS, version 9.1.
RESULTS
Subjects
Demographic characteristics, hormone levels, state anxiety, and severity of PMDD symptoms across the menstrual cycle are shown in Table 2 for the 14 women in each group who had a measurable startle response to the acoustic startle probe. One of the 15 women recruited into the PMDD group had no measurable startle response and, thus, data from this subject was dropped from all analyses. One of the 14 remaining women with PMDD underwent startle testing in the follicular phase only. Hormone parameters for the follicular phase (estradiol <80 pg/ml) were not met for 2 women with PMDD and for the luteal phase (progesterone levels ≥3 ng/ml) in three healthy controls. Thus, data from these phases were not included in analyses. Mean number of days between testing sessions was 17.5 (range 13–23 days) for both women with PMDD and healthy controls. All but one of the women with PMDD were documented to have had the onset of their PMDD symptoms by the time they underwent startle testing in the luteal phase. All PMDD subjects endorsed the hallmark symptom of irritability, and all but two endorsed both anxiety and depressed mood.
Table 2.
Demographic Characteristics, Hormone Levels, and Symptom Ratings for Women with PMDD and Healthy Controls
| Variables | PMDD (SD) | Healthy (SD) |
|---|---|---|
| (n=15) | (n=14) | |
| Age (years) | 34.5 (6.4) | 29.6 (6.5) |
| Age of education (years) | 16.0 (3.1) | 16.0 (1.9) |
| Age at menarche (years) | 13.0 (1.3) | 12.0 (1.3) |
| Age at the onset of PMDD (years) | 23.0 (7.6) | NA |
| (n=12) | (n=12) | |
| Follicular phase | ||
| Estradiol pg/ml | 43.3 (13.9) | 27.6 (10.8) |
| Progesterone ng/ml | 0.42 (0.34) | 0.39 (0.17) |
| DRSP total | 33.4 (12.9) | 25.0 (1.3) |
| Speilberger STAI | 29.4 (5.9) | 23.0 (4.3) |
| (n=13) | (n=11) | |
| Luteal phase | ||
| Estradiol pg/ml | 102.6 (34.5) | 99.6 (65.7) |
| Progesterone ng/ml | 8.5 (3.9) | 10.4 (3.6) |
| DRSP total | 66.1 (19.0) | 25.4 (1.4) |
| Speilberger STAI | 33.2 (5.4) | 23.0 (3.4) |
Mean age at the onset of PMDD was 23.7 years and the women with PMDD (34.5±6.4 years) were slightly, but not significantly (p=0.08), older than the healthy females (29.6±6.5). Age did not contribute or confound any of the effects reported herein. Likewise, there were no significant group differences in number of years of education or age at menarche. Three women with PMDD had a history of major depressive disorder, and three had a history of substance abuse/dependence disorder. Five women with PMDD reported a family history of affective disorders, two a history of substance abuse/dependence, and one an anxiety disorder. Two women with PMDD were adopted and did not know their family of origin.
Menstrual Cycle Modulation of Acoustic Startle Magnitude
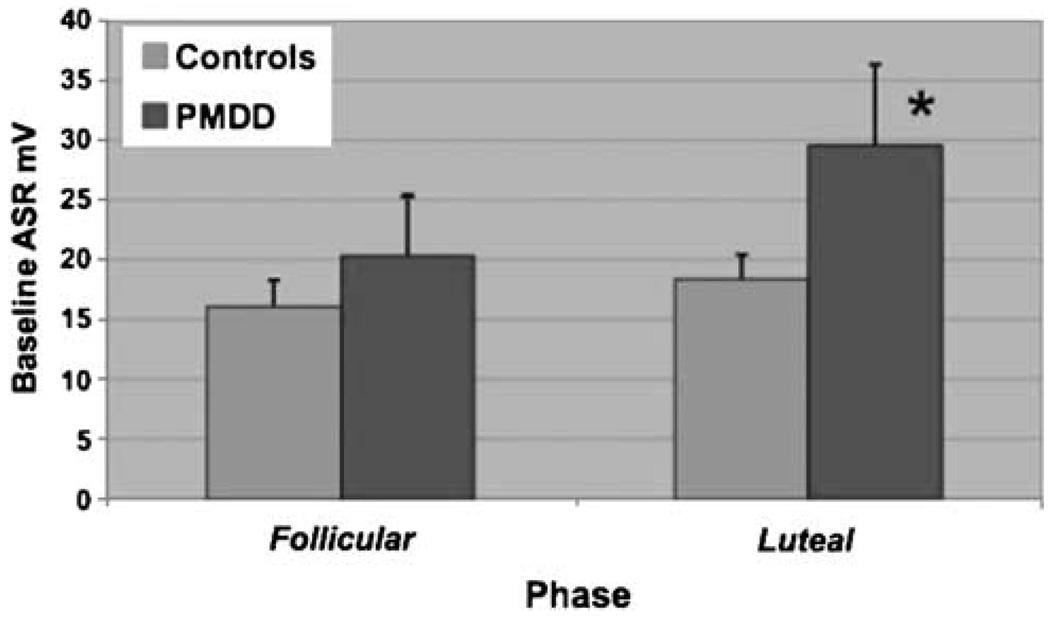
The overall phase effect for baseline ASR was significant (F1, 19.6=6.42, p=0.02). Despite a nonsignificant group–phase interaction effect (F1, 19.6=3.20, adjusted p=0.09), the phase effect is most pronounced among women with PMDD, who exhibited a significant increase in baseline ASR from the follicular to luteal phase (F1, 18.8=10.2, adjusted p=0.01). These effects are illustrated in Figure 1.
Figure 1.
Luteal-phase baseline startle is accentuated in women with PMDD. Women with PMDD experienced a significant increase in baseline ASR magnitude from follicular to luteal phase (F1, 18.8=10.2, adjusted p=0.01).
Affective Modulation of Acoustic Startle Magnitude
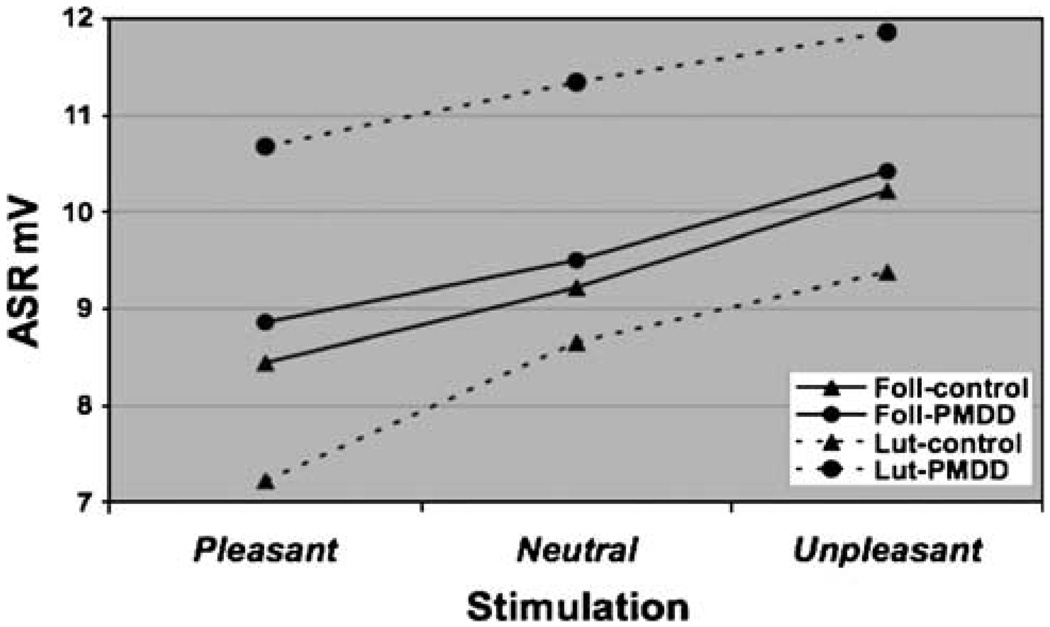
As depicted in Figure 2 for ASR, the phase, group–phase, and stimulation effects were significant (F1, 18.9=7.76, adjusted p=0.02; F1, 18.2=9.10, adjusted p=0.02; and F2, 88=17.6, adjusted p=0.0001, respectively). Interestingly, the significant group–phase interaction is driven by an overall greater startle magnitude (across stimulations) in the luteal phase compared to the follicular phase among PMDD subjects (F1, 18.9=16.3, adjusted p=0.001). By contrast, the degree to which pleasant pictures reduced and the unpleasant pictures enhanced the ASR compared to neutral pictures did not differ between groups (F2, 88=0.11, adjusted p=1.0) or in menstrual cycle phase (F2, 88=0.26, adjusted p=1.0).
Figure 2.
Affective modulation of startle. ASR–stimulation–group–phase. Results of mixed models analyses of affective modulation of the ASR revealed a significant phase (F1, 18.9=7.76, adjusted p=0.02); group–phase (F1, 18.2=9.10, adjusted p=0.02); and stimulation (F2, 88=17.6, adjusted p=0.0001). However, ASR response to stimulation did not vary by group or phase alone.
Arousal and Valence
Subject ratings of arousal were significantly greater among the pleasant and unpleasant pictures compared to the neutral pictures for all levels of phase and group. Valence scores for the pleasant, neutral and unpleasant pictures were in the expected direction with increasing scores indicating an increasing degree of unpleasantness. There were no significant phase, group–phase or group–phase–stimulation effects. Only the stimulation effect in these models was significant (F2, 108=60.0, adjusted p=0.0001 and F2, 107=290.0, adjusted p=0.0001, for arousal and valence, respectively) (Table 3).
Table 3.
Acoustic Startle Response, Arousal, and Valence Measures
| Follicular phase | Luteal phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Healthy n=12 | PMDD n=12 | Healthy n=11 | PMDD n=13 | |||||
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Acoustic startle response in mv | ||||||||
| Baseline | 16.10 | 7.60 | 20.30 | 17.70 | 18.40 | 6.65 | 29.50 | 24.10 |
| Pleasant | 8.40 | 6.00 | 8.86 | 13.26 | 7.22 | 5.16 | 10.68 | 12.59 |
| Neutral | 9.20 | 5.74 | 9.50 | 12.30 | 8.65 | 5.43 | 11.34 | 13.17 |
| Unpleasant | 10.20 | 6.69 | 10.42 | 13.05 | 9.38 | 6.05 | 11.86 | 12.95 |
| Arousal | ||||||||
| Pleasant | 3.40 | 1.01 | 4.60 | 2.15 | 5.12 | 1.08 | 4.52 | 1.63 |
| Neutral | 2.50 | 1.30 | 2.90 | 1.06 | 2.23 | 1.75 | 2.26 | 1.59 |
| Unpleasant | 6.10 | 1.15 | 4.94 | 1.55 | 5.42 | 0.99 | 5.56 | 1.02 |
| Valence | ||||||||
| Pleasant | 2.59 | 0.84 | 2.97 | 1.20 | 2.52 | 1.08 | 2.77 | 1.01 |
| Neutral | 4.92 | 0.64 | 5.14 | 0.43 | 5.61 | 1.76 | 5.44 | 1.30 |
| Unpleasant | 8.18 | 0.75 | 7.54 | 1.14 | 8.01 | 0.64 | 7.96 | 0.79 |
State Anxiety
For state anxiety, the group–phase interaction was not significant (F1, 22.5=2.11, p=0.16), however, there was a significant overall difference between PMDD subjects and healthy controls (F1, 23.6=32.5, p=0.0001). PMDD subjects reported higher state anxiety before the startle procedure during both the follicular (F1, 43.6=10.9, p=0.002) and luteal (F1, 43.7=27.2, p=0.0001) phase testing sessions compared to healthy control women.
Relationship between PMDD Symptoms and ASR
Spearman correlations were conducted between selected variables, chosen a priori, overall and by group. In these analyses, we compared the percent change from follicular to luteal phase for the DSRP and the ASR magnitude. There was no significant relationship between DSRP total or the mean of the six mood symptom scores and the magnitude of baseline ASR, and this finding was not altered when adjusting for possible diagnosis effects (all |r|<0.55, all adjusted p>0.28). Likewise, total DRSP scores within each phase did not correlate with baseline ASR during either the follicular or luteal phase, neither overall nor by group (all |r|<0.34, adjusted p>1.0).
Sex Hormones and ASR
Women with PMDD (54.8±31.9 pg/ml) had higher follicular phase estradiol levels (t=2.82, df=24, p<0.01) than healthy controls (27.6±10.8 pg/ml), although both means were well within the early to mid-follicular phase range. Estradiol during the luteal phase and progesterone during both the luteal and follicular phase were similar between groups (all p>0.58). There was no significant correlation between baseline ASR magnitude and plasma estradiol and progesterone levels in either group or in either phase (all |r|<0.65, all adjusted p>0.12).
DISCUSSION
The principal finding from this study is that PMDD is associated with a clear increase in baseline startle magnitude in the luteal phase compared to the follicular phase, while healthy female controls do not show cyclic changes in this measure of physiologic arousal. Although our finding in women with PMDD is novel, results from the healthy controls in this study are consistent with those from other laboratories (Swerdlow et al, 1997; Jovanovic et al, 2004). Given the heightened baseline startle and the negative emotional state of women with PMDD during the luteal phase, our finding that affective modulation of the ASR magnitude is intact and consistent in women with PMDD is intriguing. Together these findings suggest that, for women with PMDD, the luteal phase is a period of physiologic hyperarousal that may serve to magnify the intensity of emotional states without altering the valence of these affective states.
Several factors may have contributed individually or in concert to these findings. As mentioned previously, an organism’s emotional and perceptual state has a dramatic and well-documented impact on the ASR, with pleasant states diminishing and adverse states accentuating, the amplitude of the eye blink (Davis et al, 1997; reviewed by Koch, 1999; Lang et al, 1990, Schmid et al, 1995). Our PMDD subjects were symptomatic during the luteal-phase startle study when their ASR magnitude was most pronounced. However, the relatively asymptomatic (with respect to their primary PMDD symptoms) follicular phase was associated with a baseline ASR that was similar to that seen in healthy controls. This state-dependent accentuation of baseline startle in women with PMDD is similar to that seen in individuals with posttraumatic stress disorder (PTSD) when studied at baseline and then under conditions of contextual threat. Although exaggerated startle response is one of the symptoms of PTSD, a number of studies have found a normal (Grillon et al, 1996; Orr et al, 1997) baseline startle when individuals with PTSD are studied in ‘safe’ conditions. However, when placed in the dark or in the context of threat of electric shock, the ASR magnitude is more consistently accentuated (Morgan et al, 1995).
This distinction is important as the ASR increases, seen with anxious anticipation and/or stress arising from a contextual threat, are thought to be mediated by input from the bed nucleus of the stria terminalis (BNST; Davis and Shi, 1999). The BNST is a basal forebrain structure that is highly responsive to gonadal steroids (Isgor et al, 2002; Auger and Vanzot, 2006), is rich in GABAergic neurons (Herman et al, 2004) and glutamatergic projections (Dumont and Williams, 2004). A recent study in rodents suggests that fear-potentiated startle, which is mediated by the amygdala, is not modulated by estrogen, progesterone or allopregnanolone (ALLO). However, in a rodent model of stress/anxiety, ALLO and progesterone (presumably via ALLO) reversed the ASR accentuating effects of corticotropin releasing hormone (CRH) infused into the BNST (Toufexis et al, 2004). Compared to healthy controls and to themselves in the follicular phase, the PMDD subjects in our study were significantly more anxious/tense and irritable during the luteal phase and, as such, did not appear to require any external provocation (unpleasant picture viewing) to exhibit a heightened arousal. That we did not find a significant correlation between physiologic (ASR magnitude) and behavioral (STAI and DRSP) measures is consistent with a number of other startle studies (Dichter et al, 2004; Grillon et al, 1996, 1998a), and may reflect an insufficient sample size rather than a true absence of a relationship. Alternatively, startle and subjective ratings may reflect distinct processes. Startle is a low-level response and verbal report of anxiety involves more elaborate cognitive activity. This latter explanation is consistent with findings that the amygdala can be activated without alteration in conscious mood (Morris et al, 1998).
Menstrual cycle fluctuations in neuroactive steroids could also contribute directly to alterations in the ASR by modulating neurotransmitters and/or brain regions involved in the startle reflex (Koch, 1999) or the emotional modulation thereof (Van Nobelen and Kokkinidis, 2006). Through a series of elegant experiments, Gulinello and colleagues have demonstrated that timing and length of progesterone and ALLO exposure or withdrawal are critical with respect to the anxiolytic properties of ALLO and the pharmacologic profile of the GABAA receptor (Gulinello et al, 2002, 2003; Gulinello and Smith, 2003). Acute (minutes) and long-term (>5 days) administration of progesterone or ALLO is anxiolytic in male and female rodents (according to behavior in the elevated plus maze task), and does not lead to increases in α4 GABAA receptor subunit configuration which is associated with diminished benzodiazepine sensitivity (Gulinello and Smith, 2003). However, short-term administration (48 h) leads to an increase in anxiety, α4 subunit expression, and benzodiazepine insensitivity. Pertinent to our present study, female rodents exposed to short-term administration (Gulinello and Smith, 2003) or withdrawal (Gulinello et al, 2003) of either steroid demonstrate accentuation of the ASR that was correlated with anxiety-like behavior in the elevated plus maze (Gulinello and Smith, 2003).
That the length of progesterone and ALLO exposure may be critical with respect to its modulation of the ASR magnitude is interesting in light of our present data and those from Swerdlow et al (1997), indicating that the acoustic startle magnitude remains consistent across the highly divergent neuroendocrine milieu of the menstrual cycle in healthy human females. It is difficult to extrapolate from rodent to human the exact duration of exposure necessary to make the switch between the anxiolytic and anxiogenic profiles of ALLO. However, one could assume that our healthy subjects who were studied a mean of 5.5 days post-ovulation were captured during the ‘chronic’ phase of progesterone/ALLO exposure. Nevertheless, both our PMDD and healthy control subjects were studied a similar number of days post-ovulation and had similar luteal-phase progesterone levels. Thus timing in relationship to ovulation and/or quantitative differences in peripheral progesterone concentrations cannot explain the disparate findings in ASR magnitude between women with PMDD and healthy controls.
Instead, we suggest that luteal-phase accentuation of the ASR magnitude in women with PMDD reflects the aberrant interaction between neuroactive steroids and brain function. This neuroendocrine milieu contributes to the emergence of PMDD symptoms (Schmidt et al, 1998) and what appears to be the phase-specific heightening of physiologic arousal. The notion that women with PMDD demonstrate altered central nervous system response to normal menstrual cycle-related fluctuations in neuroactive steroids is supported by the findings of numerous other groups using a wide range of experimental methods including hormonal manipulations (Schmidt et al, 1998), transcranial magnetic stimulation (TMS; Smith et al, 2003), and pharmacologic challenges (Sundstrom et al, 1998, 1999). Women with PMDD show a depressive response to administration of exogenous ovarian steroids (Schmidt et al, 1998), demonstrate a luteal phase-specific decrease in neuronal inhibition and enhanced facilitation with TMS (Smith et al, 2003), and exhibit a luteal-phase subsensitivity to GABAA receptor agonist administration (Sundstrom et al, 1998, 1999; Sundstrom-Poromaa et al, 2003). Together with the finding that menstrual cycle-related fluctuations in occipital cortex GABA concentrations are altered in women with PMDD (Epperson et al, 2002), there is a growing body of literature in humans that implicates GABAergic dysregulation in the pathogenesis of PMDD and perhaps the physiologic hyperarousal seen here in women with PMDD during the luteal phase.
In addition to direct modulation of brain systems involved in the ASR, neuroactive steroids may have indirect effects on the ASR via the hypothalamic pituitary adrenal (HPA) axis which mediates the organism’s stress response. Interestingly, GABAergic neurons in the BNST as well as preoptic area and hypothalamus can inhibit outflow from the parvocellular paraventricular nucleus neurons and, thus, reduce adrenocorticotropin hormone (ACTH) secretion (Tringali et al, 2004; Herman et al, 2004). Although estrogen and progesterone have been shown in some studies to enhance and diminish HPA axis activation (Fonseca et al, 2001; Patchev et al, 1994), respectively, ALLO concentrations in plasma and brain increase as a result of stress (Barbaccia et al, 1997, 2001), perhaps as a compensatory mechanism. Women with PMDD fail to demonstrate the normal increase in ALLO seen in healthy subjects under conditions of stress (Monteleone et al, 2000; Girdler et al, 2001; Droogleever Fortuyn et al, 2004; Lombardi et al, 2004), or during HPA axis activation with ACTH administration (Genazzani et al, 1998; Lombardi et al, 2004). In the context of this study, the acoustic startle procedure can be viewed as mildly stress inducing. However, without cerebral spinal fluid and/or plasma levels of ALLO both immediately before and after the procedure, we were not able to determine whether the procedure was sufficiently stressful to increase ALLO production in healthy controls or whether women with PMDD lacked this response.
Between-group differences in the perception of stress or the number of stressors in the weeks surrounding each of the test sessions was not directly assessed. However, several studies indicate that women with significant premenstrual mood symptoms report life events as more stressful and/or less positive during the premenstruum than compared to the follicular phase of the menstrual cycle (Gallant et al, 1992; Woods et al, 1998), although they do not experience an increased frequency of stressful life events compared to healthy menstruating women or males (Schmidt et al, 1990; Lewis, 1995). Thus, it is the perception of events as more stressful that could render women with PMDD in a more aversive state and heighten their baseline response to the startle procedure. However, if the enhanced baseline ASRs were merely a matter of negative perceptions or affect, we would have anticipated that women with PMDD would have had an altered response to affective modulation of the ASR magnitude.
Although our findings in women with PMDD of a state and menstrual cycle phase-dependent heightening of baseline ASR in the presence of intact affective modulation of ASR are intriguing, lessons learned from the growing acoustic startle literature suggest that these findings are preliminary until replicated. However, these findings begin to address the proverbial chicken or the egg question; namely whether the negative mood state itself or the abnormal neuroendocrine milieu that mediated the negative mood state of the luteal phase alters the ASR physiology. The intact affective modulation of the ASR seen in women with PMDD in this study would suggest that the latter is more critical in PMDD. Studies in which women also undergo fear-potentiated startle would help to tease apart brain areas that may be more or less responsive to gonadal hormone and neurosteroid fluctuations in women with PMDD, and lead to further knowledge regarding the complex pathogenesis of this disorder. In addition, a measure of perceived stress of the procedure and/or peristudy life events would contribute valuable information regarding the potential impact of menstrual cycle- and/or diagnosis-specific changes in stress on physiologic arousal.
ACKNOWLEDGEMENTS
We acknowledge Shani Osbourne and Jeffrey Hille for assisting with patient recruitment/clinical care and the startle procedure, respectively, and Sobia Sarmast for her assistance in preparing the manuscript. This work was funded by a Mentored Patient-Oriented Career Development Award (K23) MH1830 (CNE), an Independent Investigator Career Award (KO2) MH73090 (CNE), an RO1 MH64845 (CNE) from the National Institute of Mental Health, an investigator award from the Dana Foundation (CNE), and support from the Intramural Research Program of the National Institute of Mental Health (CG). We also gratefully acknowledge Church & Dwight Co., Inc., Princeton, NJ for their generous donation of ANSWER® Ovulation Test kits.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Auger CJ, Vanzot RJ. Progesterone treatment of adult male rats suppresses arginine vasopressin expression in the bed nucleus of the stria terminalis and the centromedial amygdala. J Neuroendocrinol. 2006;18:187–194. doi: 10.1111/j.1365-2826.2005.01400.x. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, et al. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br J Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Pluchino N, Begliuomini S, Lenzi E, Palumbo M, Luisi M, et al. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol. 2004;19:344–353. doi: 10.1080/09513590400018223. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Pictures as prepulse: attention and emotion in startle modification. Psychophysiology. 1993;30:541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Cook EW, Hawk LW, Davis TL, Stevenson VE. Affective individual differences and startle reflex modulation. J Abnormal Psychol. 1991;100:5–13. doi: 10.1037//0021-843x.100.1.5. [DOI] [PubMed] [Google Scholar]
- Davis M, Gilmore RL. Anatomic and physiologic substrates of emotion in an animal model. J Clin Neurophysiol. 1998;15:378–387. doi: 10.1097/00004691-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann NY Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker D, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc London B Biol Sci. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early-and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41:433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Droogleever Fortuyn HA, van Broekhoven F, Span PN, Backstrom T, Zitman FG, Verkes RJ. Effects of PhD examination stress on allogrenanolone and cortisol plasma levels and peripheral benzodiazepine receptor density. Psychoneuroendocrinology. 2004;29:1341–1344. doi: 10.1016/j.psyneuen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Harrison W. Daily Rating of Severity of Problems Form. New York, NY: Department of Research Assessment and Training, New York State Psychiatric Institute; 1990. [Google Scholar]
- Endicott J, Nee J, Harrison W. Daily record of severity of problems (DRSP): reliability and validity. Arch Women’s Mental Health. 2006;9:41–49. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga KK, Mason GF, Sellers E, Gueorguieva R, Zhang W, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Wisner KL, Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom Med. 1999;61:676–697. doi: 10.1097/00006842-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Eriksson O, Backstrom T, Stridsberg M, Hammarlund-Udenaes M, Naessen T. Differential response to estrogen challenge test in women with and without premenstrual dysphoria. Psychoneuroendocrinology. 2006;31:415–427. doi: 10.1016/j.psyneuen.2005.10.004. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- Fonseca E, Basurto L, Velazquez S, Zarate A. Hormone replacement therapy increases ACTH/dehydroepiandrosterone sulfate in menopause. Maturitas. 2001;39:57–62. doi: 10.1016/s0378-5122(01)00192-x. [DOI] [PubMed] [Google Scholar]
- Gallant SJ, Popiel DA, Hoffman DM, Chakraborty PK, Hamilton JA. Using daily ratings to confirm premenstrual syndrome/late luteal phase dysphoric disorder. Part I. Effects of demand characteristics and expectations. Psychosom Med. 1992;54:149–166. doi: 10.1097/00006842-199203000-00003. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernadi F, Casarosa E, Salvestroni C, Tonetti A, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pederson CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatry. 1998a;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with PTSD. Psychiatry Res. 1996;64:169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS. Progesterone withdrawal increases the alpha4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology—a comparison with female rats. Neuropharmacology. 2002;43:701–714. doi: 10.1016/s0028-3908(02)00171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the alpha4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci. 2003;17:641–648. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- He S, Huang J, Wu X, Li L. Glutamate and GABAB transmissions in lateral amygdala are involved in startle-like electromyographic (EMG) potentiation caused by activation of auditory thalamus. Neurosci Lett. 2005;374:113–118. doi: 10.1016/j.neulet.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocorticol stress integration. Ann NY Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Tamhane AC. Multiple Comparisons Procedures. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- Isgor C, Huang GC, Akil H, Watson SJ. Correlation of estrogen β-receptor messenger RNA with endogenous levels of plasma estradiol and progesterone in the female rat hypothalamus, the bed nucleus of stria terminalis and the medial amygdala. Mol Brain Res. 2002;106:30–41. doi: 10.1016/s0169-328x(02)00407-2. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, et al. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41:401–406. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. E motion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, FL: University of Florida; 2005. International affective picture system (IAPS): digitized photographs, instruction manual and affective ratings. [Google Scholar]
- Lewis LL. One year in the life of a woman with premenstrual syndrome: a case study. Nurs Res. 1995;44:111–116. [PubMed] [Google Scholar]
- Lombardi I, Luisi S, Quirici P, Monteleone F, Bernardi F, Liut M, et al. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol Endocrinol. 2004;18:79–87. doi: 10.1080/09513590310001652955. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- Morgan CA, III, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- N-Wihlback AC, Sundstrom-Poromaa I, Backstrom T. Action by and sensitivity to neuroactive steroids in menstrual cycle related CNS disorders. Psychopharmacology. 2006;186:388–401. doi: 10.1007/s00213-005-0185-2. [DOI] [PubMed] [Google Scholar]
- Orr SP, Solomon Z, Peri T, Pitman RK, Shalev AY. Physiologic responses to loud tones in Israeli veterans of the 1973 Yom Kippur War. Biol Psychiatry. 1997;41:319–326. doi: 10.1016/s0006-3223(95)00671-0. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Schmid A, Koch M, Schnitzler HU. Conditioned pleasure attenuates the startle response in rats. Neurobiol Learning Memory. 1995;64:1–3. doi: 10.1006/nlme.1995.1037. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Grover GN, Hoban MC, Rubinow DR. State-dependent alterations in the perception of life events in menstrual-related mood disorders. Am J Psychiatry. 1990;147:230–234. doi: 10.1176/ajp.147.2.230. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. New Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry. 2003;54:757–762. doi: 10.1016/s0006-3223(02)01924-8. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory: A Comprehensive Bibliography. Palo Alto, CA: Consultant Psychologists Press; 1984. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R-Non-Patient Edition (SCID-NP, Version 1.0) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Sundstrom I, Andersson A, Nyberg S, Ashbrook D, Purdy RH, Backstrom T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology. 1998;67:126–138. doi: 10.1159/000054307. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Backstrom T, Wang M, Olsson T, Seippel L, Bixo M. Premenstrual syndrome, neuroactive steroids and the brain. Gynecol Endocrinol. 1999;13:206–220. doi: 10.3109/09513599909167557. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch Women’s Mental Health. 2003;6:23–41. doi: 10.1007/s00737-002-0147-1. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Davis C, Hammond A, Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J Neurosci. 2004;24:10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tringali G, Aubry J, Moscianese K, Zamori C, Vairano M, Preziosi P, et al. Valproic acid inhibits corticotropin-releasing factor synthesis and release from the rat hypothalamus in vitro: evidence for the involvement of GABAergic neurotransmission. J Psychiatry Neurosci. 2004;29:459–466. [PMC free article] [PubMed] [Google Scholar]
- Van den Buuse M, Eikelis N. Estrogen increases prepulse inhibition of acoustic startle in rats. J Pharmacol. 2001;425:33–41. doi: 10.1016/s0014-2999(01)01139-6. [DOI] [PubMed] [Google Scholar]
- Van Nobelen M, Kokkinidis L. Amygdaloid GABA, not glutamate neurotransmission or mRNA transcription controls footshock-associated fear arousal in the acoustic startle paradigm. Neuroscience. 2006;137:707–716. doi: 10.1016/j.neuroscience.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Woods NF, Lentz MJ, Mitchell ES, Heitkemper M, Shaver J, Henker R. Perceived stress, physiological stress arousal, and premenstrual symptoms: group differences and intra-individual patterns. Res Nurs Health. 1998;21:511–523. doi: 10.1002/(sici)1098-240x(199812)21:6<511::aid-nur5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]