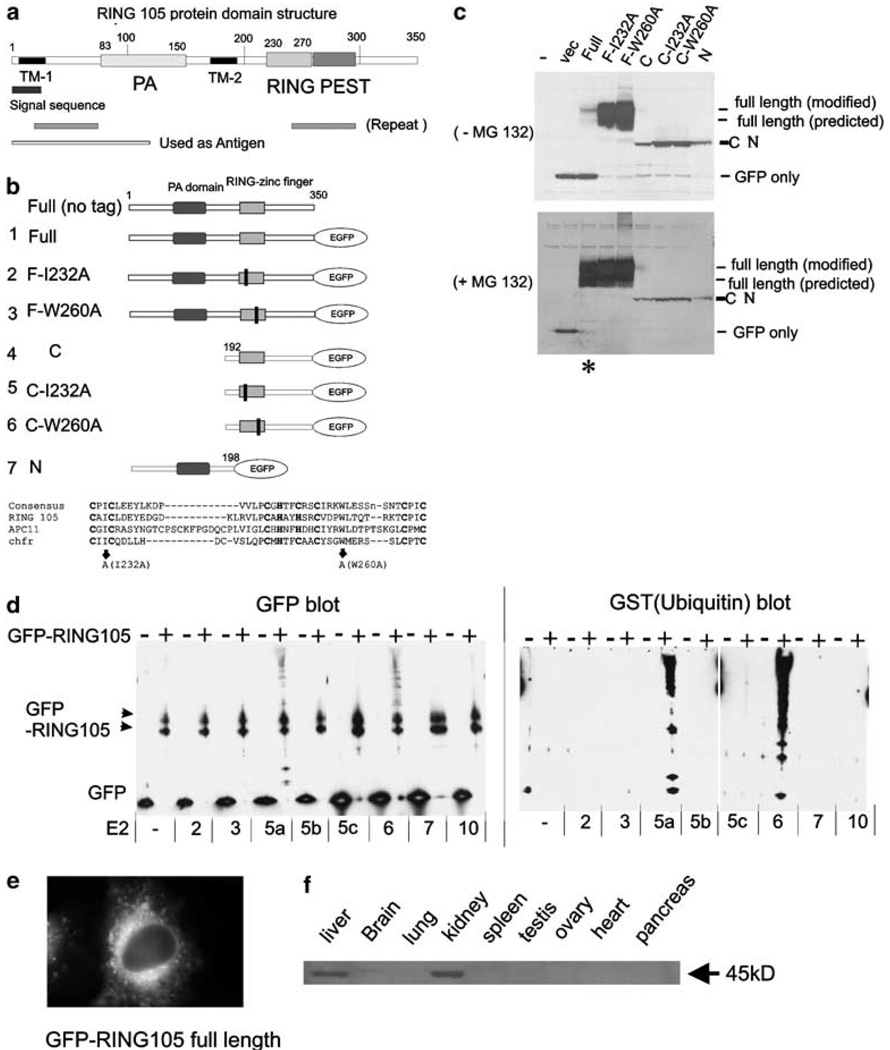
Figure 1. RING105 is a RING-finger-containing ubiquitin ligase.
(a) RING105 protein domain structure. RING105 protein is 350 amino acids and contains a PA (protease-associated) domain, a RING-finger domain and a PEST domain. Two TM (transmembrane domain) are also highlighted. (b) Plasmid constructs. RING105 was truncated, or point mutations (represented as black bars) were introduced in conserved positions within the RING-finger domain. Mutations at corresponding sites were previously shown to inactivate ubiquitylation activity in the chfr protein (Kang et al., 2002). The protein sequence alignment comparing RING-finger domains for RING105, Apc11 and chfr is also shown. The residues that were mutated to inactivate the RING finger are indicated by arrows. The cysteine and histidine residues that form zinc-chelating ‘fingers’ are shown in bold. (c) RING105 is degraded by the proteasome in a manner that is dependent on its own RING-finger domain. 293T cells were transfected with plasmids encoding the indicated GFP-tagged RING105 constructs and cell extracts were prepared for immunoblotting with anti-GFP. In the lower blot, the cells were treated with proteasome inhibitor MG132 (10 µm) 2 h before preparation of cell extracts. Note that mutants containing point mutations within the RING finger are stable (compare lane 3 with lanes 4 and 5 in the upper blot). Note also that MG132 treatment results in stabilization of the full-length RING105 protein (compare lane 3 (asterisk) in the upper and lower blots). (d) RING105 has autoubiquitylation activity in vitro when tested with UbcH5a and UbcH6. Immunoprecipitated GFP- or GFP-tagged RING105 were incubated with indicated E2 (ubiquitin-conjugating enzyme), GST-ubiquitin, E1 and ATP for 60 min at 30°C. After the reaction, the beads were rinsed extensively, then treated with SDS sample buffer, separated by PAGE and transferred to membranes. They were then probed with anti-GFP (left panel) and anti-GST (ubiquitin) (right panel). The high molecular weight bands appearing above the GFP-RING105 doublet (black arrowheads) when RING105 is incubated in conjunction with UbcH5a and UbcH6 reflect polyubiquitylated proteins. (e) GFP-tagged RING105 associated with cytoplasmic membranes. HeLa cells were transfected with GFP-RING105 (full) (construct 1). After 18 h, cells were treated with 10 µm MG132 for 2 h and observed. (f) RING105 is enriched in the liver and kidney. Extracts from human tissues were probed with anti-RING105 antibody. Further description of the antibody is shown in Supplementary Information 1.