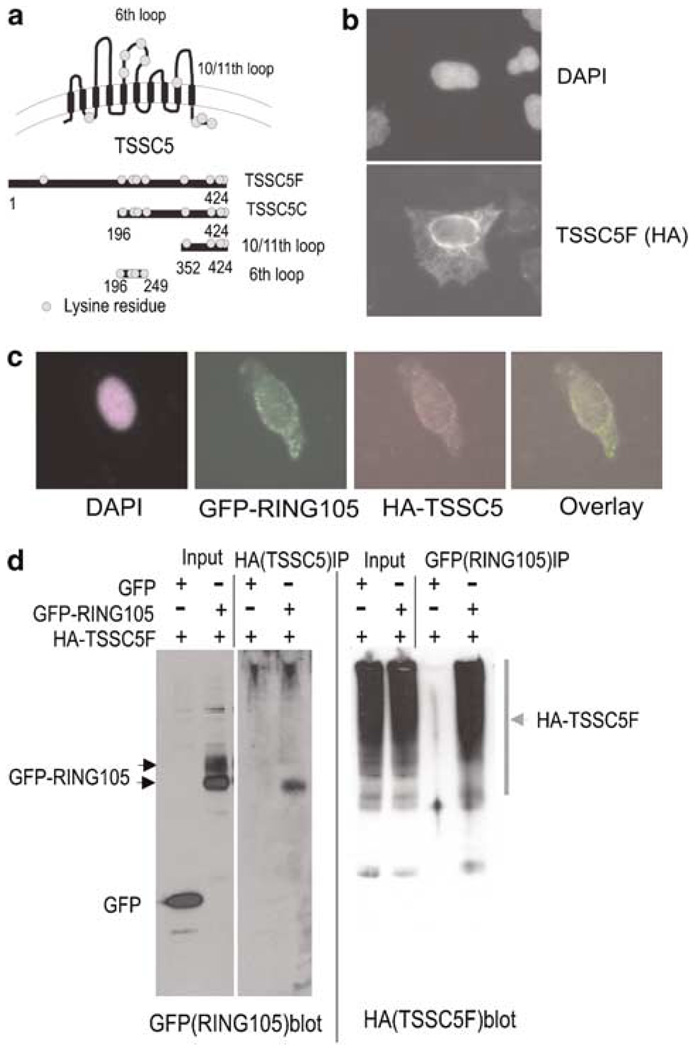
Figure 2. RING105 binds TSSC5 by co-immunoprecipitation.
(a) Schematic representation of TSSC5 protein and TSSC5 plasmid constructs used for testing ubiquitin targeting. TSSC5 is predicted to be an integral membrane protein with 10 transmembrane domains. (The orientation of TSSC5 with respect to its insertion into cytoplasmic membranes such as ER has not been determined.) Potential ubiquitin acceptor lysine residues are presented as circles. (b) Ectopically expressed TSSC5 was associated with cytoplasmic membranes. HeLa cells were transfected with HA-tagged TSSC5F (full length) and prepared for immunofluorescence with anti-HA antibody. DNA was stained with DAPI (upper panel). (c) Limited colocalization of GFP-RING105 and HA-TSSC5F. GFP-RING105 and HA-TSSC5F (full length) were cotransfected to HeLa cells. At 14 h after transfection, cells were treated with MG132 for 2 h to prevent RING105 degradation, and fixed for localization analysis. Panels represent DAPI (purple), GFP-RING105 (green) and HA-TSSC5F (red) and GFP-HA overlay. (d) HA-tagged TSSC5 co-immunoprecipitated with GFP-tagged RING105. HeLa cells were transfected with HA-TSSC5F (full length) and GFP or GFP-RING105. After 18 h, cells were treated with MG132 for 2 h and then extracted. The extracts were immunoprecipitated with anti-HA and anti-GFP. Samples from the whole extract (input) and the immunoprecipitate (IP) were probed with anti-HA and anti-GFP.