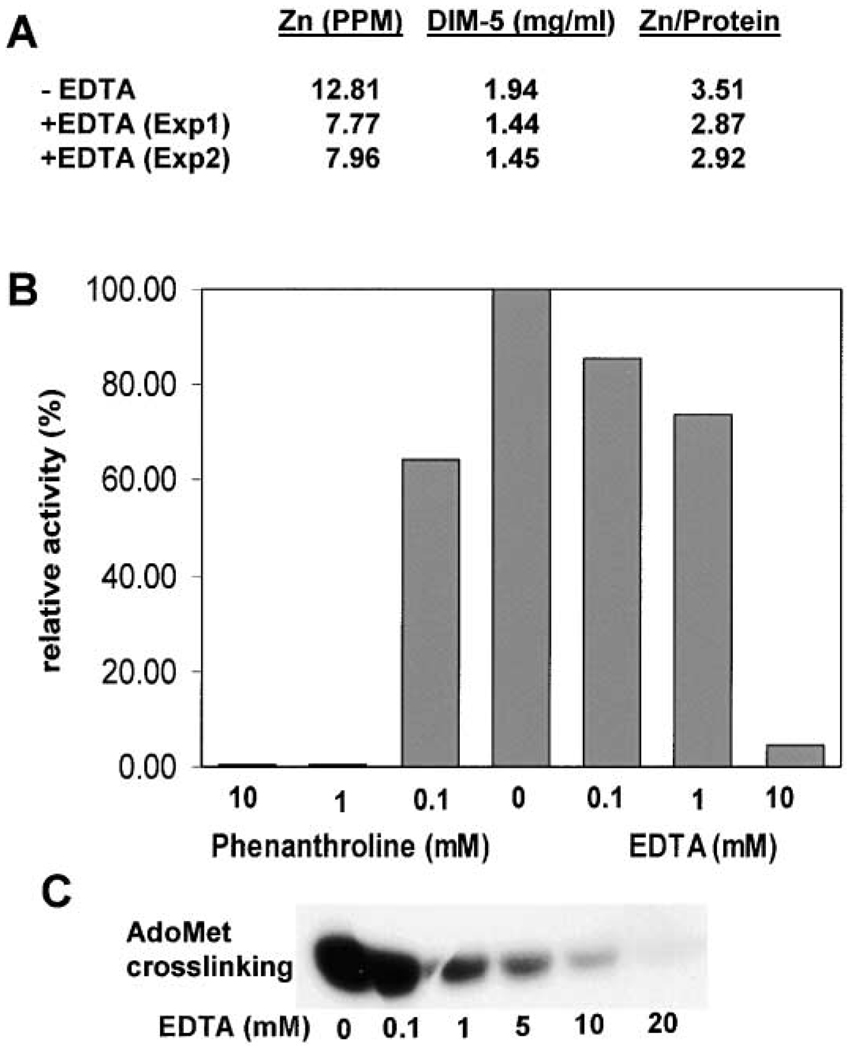
Figure 6. Metal Chelators Inhibit DIM-5 Activity.
(A) Analysis of zinc content of DIM-5 with and without EDTA treatment. DIM-5 protein was incubated with 20 mM EDTA for 2 days, at which time HKMT activity was no longer detectable. To remove zinc bound to EDTA, the protein was either dialyzed (Exp1) or subjected to gel filtration chromatography (Exp2) against 20 mM glycine (pH 9.8), 5% glycerol, 0.5 mM DTT, and 1 mM EDTA.
(B) Purified DIM-5 protein (1 mg/ml in 20 mM glycine [pH 9.8], 5% glycerol) was incubated with various concentration of 1,10-phenanthroline or EDTA for 18 hr at 4°C. The enzyme was diluted 80-fold and assayed for HKMT activity under standard conditions, except that no DTT was present.
(C) Fluorographic results of AdoMet crosslinking in the presence of EDTA.