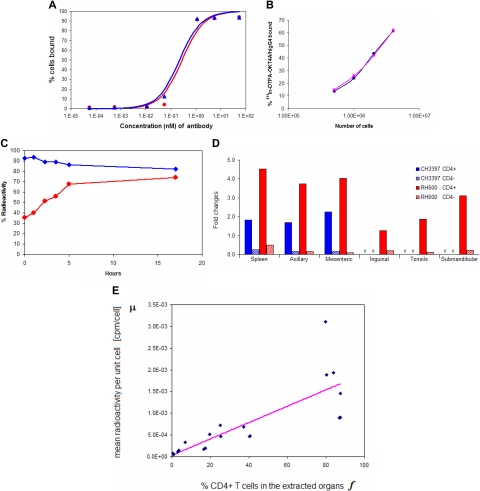
Figure 1.
Binding affinity and immunoreactivity of OKT4A/hIgG4 and 111In-DTPA- OKT4A/hIgG4 to MT4 cells. (A) Assay based on constant cell concentration. Percentage of cells-bound mAb was plotted vs increasing concentrations of OKT4A/hIgG4 (red circles) or DTPA-OKT4A/hIgG4 (blue triangles) mAb. (B) Assay based on constant 111In-DTPA-OKT4A/hIgG4 concentration. The percentage of 111In-DTPA-OKT4A/hIgG4 bound to MT4 cells was plotted vs the number of MT4 cells. The specific activity of the labeled antibody was more than 11 mCi/mg. The labeled antibody with a radiochemical purity of more than 98% was used for the cell-binding and the imaging studies. (C) The radiotracer retention and internalization assays were performed in MT4 cells warmed at 37°C in a humidified 5% CO2 incubator. The percentage of 111In-DTPA-OKT4A/hIgG4 bound to MT4 cells (blue) was obtained by dividing the radioactivity associated with cell pellets by the total radioactivity measured in 1-mL aliquots of cells suspensions; the internalized cell-associated fraction was determined with an acid wash method10,11 (red). (D) Ratios (fold changes) of the cpm/CD4+ over cpm/cell and cpm/CD4− over cpm/cell. Extracted total cell suspensions were obtained from spleen and lymph nodes of CH3397 and RH600 after lysing erythrocytes, and the total cell pellet counted in the gamma counter. CD4+ cells were isolated with magnetic beads and the total radioactivity associated with the CD4+ and CD4− cells determined (expressed as cpm). Ratios (fold changes) of the cpm/CD4+ over cpm/cell and cpm/CD4− over cpm/cell are shown for positive and negative selections of cells purified with magnetic beads from organs extracted from CH3397 and RH600. x indicates not available. (E) Best fit to the linear model in Equation 2 of the data collected from unpurified, positive and negative selections of cells extracted from organs of RH600.