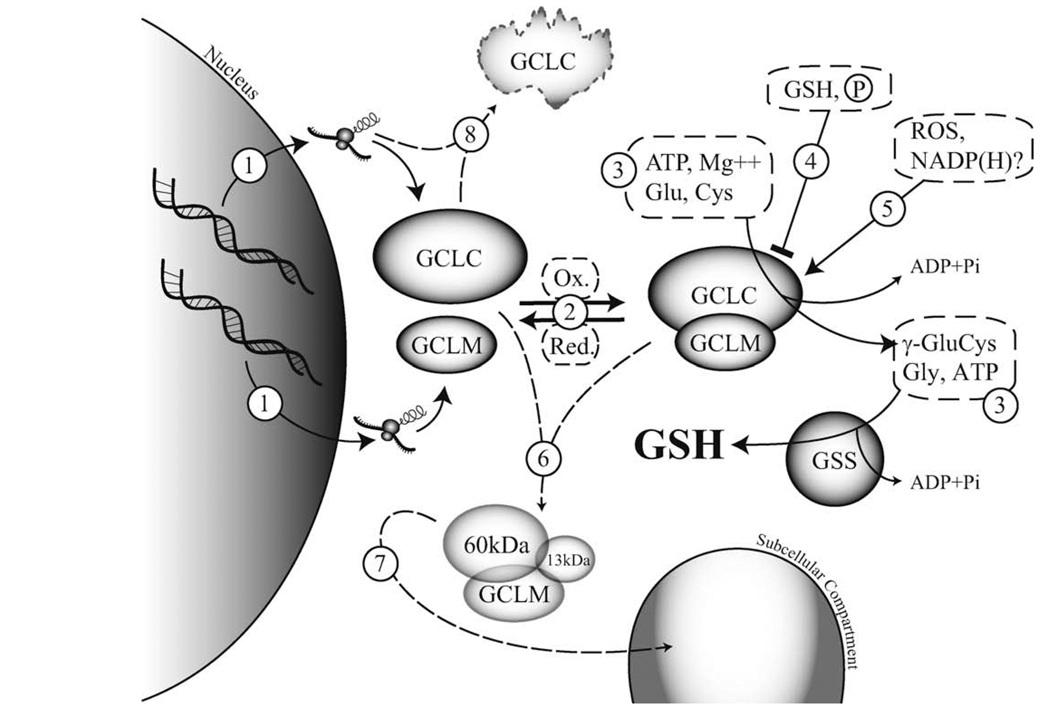
Fig. 2.
Post-translational regulation of glutamate cysteine ligase activity. Glutathione (GSH) is synthesized by a two-step ATP-dependent process. The first and rate-limiting step is the formation of γ-glutamylcysteine (γ-GluCys) by glutamate cysteine ligase (GCL), which is composed of catalytic (GCLC) and modifier (GCLM) subunits. The second step is the ligation of glycine (Gly) to γ-GluCys, which is catalyzed by glutathione synthetase (GSS), to form GSH. Cellular regulation of GCL activity can occur by a number of mechanisms. (1) Transcriptional or post-transcriptional: activation or suppression of gene transcription [Sections 2, 4.1 and 4.6] and/or mRNA stability, and intra- and 5′UTR GCLC polymorphisms [Sections 4.1 and 5]. (2) Reversible GCL holoenzyme formation: redox-sensitive [Section 4.1], dependent on molar ratios of GCL subunits (GCLM is usually limiting) [Section 4.1], subunit stability [Section 4.6]. (3) Substrate (Glu, Cys, Gly) and co-factor (ATP, Mg++) availability [Section 1]. (4) Inhibition of GCLC activity: negative feedback regulation by GSH [Sections 1 and 4.1], autophosphorylation and kinase-mediated phosphorylation of GCLC [Section 4.2]. (5) Activation of GCL: oxidative stress [Section 4.1], NADPH (activation or inhibition) [Section 4.3]. (6) Caspase-mediated cleavage of GCLC [Sections 4.4 and 4.6]. (7) Myristoylation of the 13 kDa C-terminal fragment of caspase-cleaved GCLC and/or altered subcellular localization [Section 4.5]. (8) Inducible or mutational regulation of GCLC protein stability [Section 4.6].