Abstract
The flavonoid quercetin suppresses cell proliferation and enhances apoptosis in vitro. In this study, we determined whether quercetin protects against colon cancer by regulating the protein level of phosphatidylinositol 3-kinase (PI 3-kinase) and Akt or by suppressing the expression of proinflammatory mediators [cyclooxygenase (COX)-1, COX-2, inducible nitric oxide synthase (iNOS)] during the aberrant crypt (AC) stage. Forty male rats were randomly assigned to receive diets containing quercetin (0 or 4.5 g/kg) and injected subcutaneously with saline or azoxymethane (AOM; 2 times during wk 3 and 4). The colon was resected 4 wk after the last AOM injection and samples were used to determine high multiplicity AC foci (HMACF; foci with >4 AC) number, colonocyte proliferation and apoptosis by immunohistochemistry, expression of PI 3-kinase (p85 and p85α subunits) and Akt by immunoblotting, and COX-1, COX-2, and iNOS expression by real time RT-PCR. Quercetin-fed rats had fewer (P = 0.033) HMACF. Relative to the control diet, quercetin lowered the proliferative index (P = 0.035) regardless of treatment and diminished the AOM-induced elevation in crypt column cell number (P = 0.044) and expansion of the proliferative zone (P = 0.021). The proportion of apoptotic colonocytes in AOM-injected rats increased with quercetin treatment (P = 0.014). Levels of p85 and p85α subunits of PI 3-kinase and total Akt were unaffected by dietary quercetin. However, quercetin tended to suppress (P < 0.06) the expression of COX-1 and COX-2. Expression of iNOS was elevated by AOM injection (P = 0.0001). In conclusion, quercetin suppresses the formation of early preneoplastic lesions in colon carcinogenesis, which occurred in concert with reductions in proliferation and increases in apoptosis. It is possible the effects on proliferation and apoptosis resulted from the tendency for quercetin to suppress the expression of proinflammatory mediators.
Introduction
Except for a few notable epidemiological studies (1,2), evidence suggests that a diet rich in fruits and vegetables may protect against chronic diseases, such as colon cancer (3,4), in possibly a site-specific manner (5). Sporadic cases of colon cancer take many years to reach the invasive stage, providing opportunities to implement appropriate dietary chemoprotective measures that may prevent the onset or slow the progression of this disease (3,6). Chemoprotective agents that decrease proliferation and enhance removal of transformed cells through apoptosis (7–9) or suppress inflammation (10,11) would be able to suppress colon tumor formation (12).
Quercetin (3,5,7,3′4′ pentahydroxyflavone) is the main flavonol present in human diets (13), making it a major contributor to the bioactivity found in fruits and vegetables. Quercetin has been shown to inhibit cancer in animal models (14) and to inhibit the growth of human cancer cell lines (15,16). The colonic epithelium maintains a dynamic equilibrium between apoptosis, necrosis, and cell proliferation and defects in the regulation of proliferation and apoptosis promote tumor formation by preventing the systematic removal of genetically mutated cells (8,12,17).
One signaling pathway involved in the control over proliferation and apoptosis, which is typically dysregulated in multiple human cancers, is the phosphatidylinositol 3-kinase (PI 3-kinase)4-Akt pathway (18). Specific inositol lipids phosphorylated by PI 3-kinase control a cascade of responses that mediate cell cycle progression, cell proliferation, and apoptosis in response to mitogenic stimuli (18,19). Binding of PI 3-kinase products PtdIns(3,4)P2 and PtdIns(3,4,5)P3 to its PH domain leads to PDK1-mediated phosphorylation of Akt, inhibiting apoptosis and allowing for dysregulated growth of transformed cells (20).
PI 3-kinases are heterodimeric proteins containing both a regulatory (p85) and catalytic subunit (p110) (21). The p85 regulatory subunit is encoded by 3 genes and a mutated form of the p85α regulatory subunit of PI 3-kinase is thought to promote colon cancer development (22). PI 3-kinase expression and activity are reported to be elevated in human colorectal tumors (23) and quercetin and its analogue LY294002 have been shown to inhibit PI 3-kinase activity (24). Other studies of colon (25) and lung cancer (26) cell lines have demonstrated that quercetin inhibits the expression of Akt.
Perturbations in the expression of proinflammatory mediators, including COX-1, COX-2, and Inducible nitric oxide synthase (iNOS), are also found in colon carcinogenesis (27,28). All 3 of these enzymes are elevated during different stages of colon cancer (29,30). However, experiments using bioactive molecules (e.g. naringin) have demonstrated that the expression of these enzymes can be suppressed at the promotion stage of the azoxymethane (AOM) rat model of colon cancer (31).
Thus, this project had as its goal the determination of whether quercetin suppresses the early stage of colon carcinogenesis (preneoplastic lesion formation) and to establish whether the effects were associated with alterations in apoptosis and proliferation and changes in the expression of cell cycle regulatory kinases (PI 3-kinase and Akt) and inflammatory mediators (COX-1, COX-2, and iNOS) in a carcinogen-injected animal model.
Materials and Methods
Dietary quercetin analysis.
Methanol extracts of the diets (10 g/L) were prepared by shaking for 24 h followed by filtration through a 0.45-μm filter. A 15-μL sample of the extract was injected into a Waters Nova-Pak C-18 column (4 μm × 15 cm) using a solvent phase of 45% methanol adjusted to a pH of 2.4 with phosphoric acid. The flow rate was 1 mL/min. Quercetin was detected at 370 nm and quercetin content calculated using an external standard of capsicum extracts (32).
Rats and treatments.
Forty male weanling Sprague-Dawley rats (Harlan) were individually housed in a temperature- and humidity-controlled animal facility with a 12-h-light/-dark cycle. The rats were stratified by body weight and assigned to 1 of 4 experimental groups (n = 10 rats per group) such that there were no differences in mean initial weight among the treatment groups. Diets were prepared to conform to the standard AIN-76A formulation (9). Controls were fed a diet consisting of 60 g/kg cellulose, 150 g/kg corn oil, 220 g/kg casein, 510 g/kg dextrose and complete vitamin (11.2 g/kg) and mineral (39.1 g/kg) mixes (AIN-76A) (Harlan Teklad). Experimental rats were fed the same diet with 4.5 g/kg quercetin dihydrate (Sigma) added at the expense of dextrose. AOM (Sigma; 15 mg/kg body weight) or saline was injected (subcutaneously) 3 wk after starting experimental diets followed by a second injection 1 wk later.
Sample collection.
Four weeks after the second injection, the entire colon was resected and cleaned with RNase free PBS (Gibco). One-centimeter sections from the most proximal and distal colon were fixed in a 4% paraformaldehyde solution and another 1-cm section was removed from the proximal and distal colon and fixed in ethanol. The remaining midsection of the cleaned colon was split longitudinally with one side used for the aberrant crypt (AC) foci (ACF) assay and the other half used for immunoblotting and PCR analyses.
ACF analysis.
Colon strips were stained with 0.5% methylene blue prepared in PBS and the number of AC, ACF, and high multiplicity ACF (HMACF; ACF with >4 AC per focus) were determined according to the procedures described by Bird (33) and used previously in our laboratory (31).
Proliferation.
Cell proliferation was measured in ethanol-fixed distal colon sections by detecting proliferating cell nuclear antigen levels with a monoclonal antibody (anti PC-10; Signet Laboratories) and a Vectastain ABC Elite kit (Vector Lab) as described by Vanamala et al. (31). Twenty-five crypt columns in the distal colon of each rat were used to determine the number of proliferating cells. Only cells exhibiting strong nuclear staining were counted. The data collected included crypt height (no. of cells per crypt column), the number and proportion of proliferating cells in a crypt column, and proliferative zone (position of the highest proliferating cell, as a percentage of the total crypt column cell no.).
Apoptosis.
The proportion of apoptotic colonocytes was measured in paraformaldehyde-fixed distal colon sections using a TUNEL assay (Intergen) as previously described (8). Stained cells exhibiting apoptotic morphological features were counted in 50 crypt columns. The apoptotic index represents the proportion of cells undergoing apoptosis within a crypt column.
Quantification of PI 3-kinase subunits and total Akt by immunoblot.
The concentration of protein in rat colon mucosal triton-soluble cell lysates was determined using the BCA Protein assay (Pierce) (31). Aliquots of the protein extracts were separated on 4–12% NuPAGE Bis-Tris precast gels (Invitrogen) and transferred to Invitrolon PVDF membranes (Invitrogen). Primary antibodies acquired from Upstate (PI 3-kinase p85), Santa Cruz Biotechnology (PI 3-kinase p85α), and Cell Signaling Technology (detects Akt1, Akt2, and Akt3 proteins) along with a peroxidase-labeled secondary antibody [anti-rabbit IgG (H+L); KPL] were used to probe the blots. Target proteins were quantified with SuperSignal West Femto (Pierce) and band intensities were determined using a Bio Rad Fluor-Imager and Quantity One software (Bio-Rad). Intensities were normalized to a standard (stimulated Jurkat cell lysate, Upstate) included on each gel.
COX-1, COX-2, and iNOS expression.
The scraped mucosa samples were placed into denaturation solution (Totally RNA kit; Ambion), homogenized on ice using a Teflon-in-glass homogenizer, and frozen (−80°C) until the RNA was isolated using a Totally RNA kit followed by treatment with DNase (DNA Free; Ambion). Prior to performing PCR analyses, all samples were tested for quality using an Agilent 2100 Bioanalyzer (Agilent). PCR reactions for COX-1 (forward primer, TGGCCGAGGATGTCATCA; reverse primer, CCTCTTTCGGTATTCATTGAAGGA) and COX-2 (forward primer, GCACAAATATGATGTTCGCATTC; reverse primer, CAGGTCCTCGCTTCTGATCTG) were performed using SYBR green detection reagent (Applied Biosystems). The expression of iNOS was determined with an Assay-on-Demand (Rn99999069_mH, Applied Biosystems) target mix using the manufacturer's protocol. Expression was normalized to 18S levels (34).
Statistics.
The Proc Mixed procedure in SAS was used to determine the effect of diet, treatment, and the interaction on HMACF number, number of cells per crypt column, extent of proliferative zone, proliferative index, protein levels, and gene expression. These data are reported as least squares means (± SEM) with differences detected using Fisher's least significant difference. Nonparametric and Wilcoxon's rank sum tests in SAS were used to determine diet and treatment effects on apoptotic index. Differences were deemed significant when P-values were <0.05.
Results
The diets were formulated to contain 4.5 g/kg quercetin. Analysis of diet aliquots indicated that the actual concentration of quercetin in the diet was 4.35 g/kg. Intake and weight gain were not affected by either dietary quercetin or carcinogen injection.
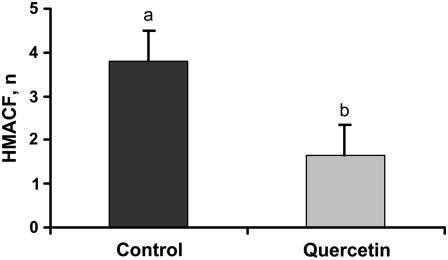
ACF are prevalent in humans and animal models of colon cancer (6,35). These preneoplastic lesions, especially the HMACF, are thought to be a predictor of eventual tumor formation (36). Therefore, we examined the prevalence of AC in these rats to determine whether quercetin was suppressing colon carcinogenesis. AC were not detected in saline-injected rats. As expected, quercetin consumption by AOM-injected rats resulted in 41% fewer HMACF than was found in rats consuming diets without quercetin (Fig. 1).
FIGURE 1 .
HMACF (foci containing >4 AC) formation in AOM-injected rats. Bars represent least squares means ± SEM, n = 10 rats. Means without a common letter differ, P = 0.033.
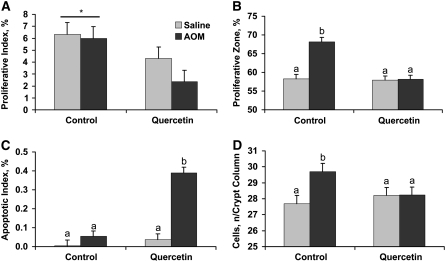
Colon cancer is characterized by elevated proliferation and reduced apoptosis; thus, we determined whether quercetin was able to mitigate these perturbations in the distal colon. We used the PCNA assay to quantify the effect of dietary quercetin on colonocyte proliferation. Rats fed quercetin, regardless of treatment, had fewer proliferating cells per crypt column (data not shown) and a lower percentage of proliferating cells per crypt column (Fig. 2A). AOM injection caused an expansion of the proliferative zone of rats consuming the control diet, which did not occur in those rats fed quercetin (Fig. 2B). We used the TUNEL assay to determine the effect of dietary quercetin on apoptosis in rat colonocytes. Quercetin yielded a greater proportion of apoptotic cells in the distal colon of carcinogen-injected rats (Fig. 2C) but had no effect on apoptosis in saline-injected rats. The net effect of changes in proliferation and apoptosis in rats consuming quercetin led to maintenance of the number of cells per crypt column when injected with the carcinogen. However, rats consuming the control diet and injected with AOM had more cells per crypt column (Fig. 2D).
FIGURE 2 .
Proliferative index (A), proliferative zone (B), apoptosis (C), and number of cells per crypt column (D) in rats injected with saline or the carcinogen AOM and fed control or quercetin-containing diets. Bars represent least squares means ± SEM, n = 10 rats. *Different from the quercetin diet, P = 0.035. Means in panels B–D without a common letter differ, P ≤ 0.044.
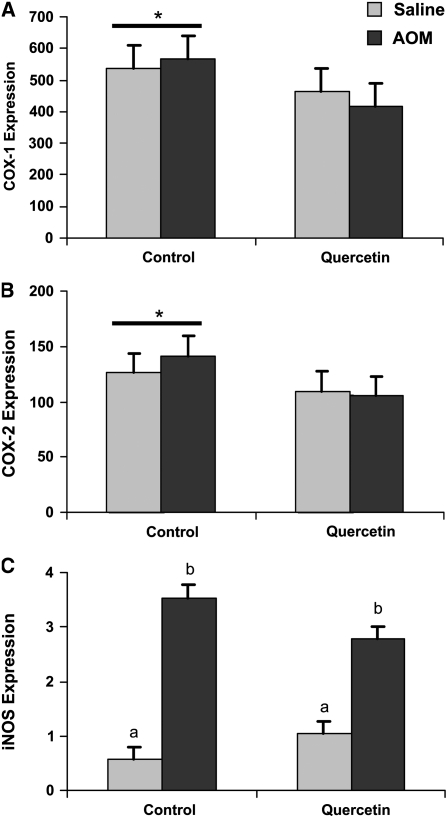
Regulation of proliferation and apoptosis is effected through inputs from a variety of signaling cascades. We chose to evaluate the effect of the carcinogen and quercetin on the level of the p85 and p85α subunits of PI 3-kinase, as well as total Akt, in colon mucosal scrapings using immunoblotting. Even though there was a consistent numerical elevation in the level of p85 (0.55 vs. 0.68; P = 0.16; SEM = 0.09), p85α (0.88 vs. 0.92; P = 0.43; SEM = 0.15), and Akt (0.09 vs. 0.22; P = 0.078; SEM = 0.08), especially in the scraped colonic mucosa from saline-injected rats fed quercetin, the steady-state level of these proteins did not differ in response to dietary quercetin (Supplemental Fig. 1). To determine whether quercetin was influencing proliferation, apoptosis, and AC formation through inflammatory pathways, we determined the mRNA levels of COX-1, COX-2, and iNOS in the scraped mucosal samples. Injection of AOM resulted in no difference (P > 0.4) in the expression of COX-1 or COX-2; however, AOM induced a 5.3-fold elevation in iNOS levels for the control rats (P = 0.0001) compared with a 1.7-fold elevation in the quercetin-fed rats (P = 0.017). Overall, quercetin suppressed COX-1 and COX-2 transcript levels by 20.2 and 19.6%, respectively (Fig. 3).
FIGURE 3 .
COX-1, COX-2, and iNOS transcript levels in colon mucosal scrapings from rats injected with saline or the carcinogen AOM and fed control or quercetin-containing diets. Bars represent least squares means ± SEM, n = 10 rats. *Tends to differ from the quercetin diet, P < 0.06. In panel C, means without a common letter differ, P < 0.0001.
Discussion
The multistep nature of colon cancer provides many opportunities to influence the disease process through nutritional interventions (37). A diet rich in fruits and vegetables that contain a variety of vitamins, trace minerals, and biologically active compounds (e.g. quercetin) may suppress carcinogenesis (13,38). In the present study, we have shown that a diet containing quercetin decreased HMACF, the number of cells per crypt column, the proliferative zone, and the percentage of proliferating cells, and increased the apoptotic index in the distal colon compared with the control diet without quercetin.
The mechanism(s) or cellular pathway(s) by which quercetin decreased the preneoplastic lesions and mitigated the effect of AOM on cell cycle kinetics does not appear to involve the transcriptional or translational regulation of PI 3-kinase or Akt. This is in contrast to data from cell culture experiments indicating that quercetin decreased the level of total Akt (25,26,39). However, other data from different cell systems suggest that quercetin has no effect on Akt expression (40), similar to results obtained in the current study. Although there are contradictory cell culture data in the literature, our current work suggests that dietary quercetin has no in vivo effect on expression of these proteins. Because the current experiment used samples collected from rats only 4 wk after the last AOM injection, it is possible the level of PI 3-kinase or Akt may not be elevated at this early time point. Therefore, it is not possible to definitively predict whether quercetin would be able to suppress expression later in tumorigenesis, a stage when the level of these proteins are typically elevated (41).
Because the activity of PI 3-kinase and Akt proteins is determined by their phosphorylation state, dietary quercetin may have altered proliferation and apoptosis by inhibiting the activity of these proteins via a mechanism that influences post-translational modification. Quercetin, as well as its structural analogue, LY294002, has been shown to inhibit PI 3-kinase activity in vitro (24,42), with the inhibition being directed at the ATP binding site. Other cell culture studies have recently demonstrated that quercetin is able to downregulate the levels of phospho-Akt (25,26).
Although the current in vivo study did not find an inhibitory effect of dietary quercetin on the expression of PI 3-kinase or its downstream effector Akt, the data do suggest that quercetin inhibits the early stage of colon carcinogenesis through other mechanisms that involve changes in both proliferation and apoptosis. Other candidate proteins that may influence the early stage of colon carcinogenesis include proinflammatory mediators, such as COX-1, COX-2, and iNOS. Takeda et al. (27) demonstrated that during early polyp development, the observed increases in COX-2 expression were dependent upon elevated COX-1 expression. Our results suggest that dietary quercetin tends to suppress the levels of both COX-1 and COX-2 expression at this very early stage of colon carcinogenesis. iNOS expression is increased in human tumors (28) and although quercetin had no significant effect on iNOS expression, it contributed toward a numerically smaller increase in iNOS levels when rats were injected with AOM. These data suggest that quercetin may suppress the promotion of colon carcinogenesis through its ability to lower proinflammatory COX gene expression during the earliest stages of colon carcinogenesis and may partially suppress the elevation in iNOS expression that occurs with carcinogen exposure.
In summary, quercetin suppressed proliferation and enhanced apoptosis and together, these changes contributed to a significant reduction in the development of ACF in the AOM model of colon carcinogenesis. Although quercetin did not influence the expression of the PI 3-kinase p85 subunits or Akt proteins, it did tend to suppress mRNA levels of COX-1 and COX-2. Early increases in iNOS expression in AOM-injected rats may promote carcinogenesis; however, it is not clear whether, at this time point, quercetin is able to suppress the increase in iNOS expression. These observations suggest that diets with elevated quantities of fruits and vegetables that provide levels of quercetin representative of that used in this study should help inhibit the formation of preneoplastic lesions of colon cancer, thus possibly reducing eventual colon tumor formation. The protection conferred by quercetin may be in part due to a suppression of proinflammatory mediators typically upregulated at disease onset.
Supplementary Material
Acknowledgments
We thank Dr. Kil Sun Yoo of the Texas A&M University Vegetable and Fruit Improvement Center for performing the diet quercetin analyses.
Supported by the Cooperative State Research, Education, and Extension Service, USDA, under agreement numbers 2001-34402-10543, 2003-34402-13647, and 2004-34402-14768, “Designing Foods for Health” through the Vegetable and Fruit Improvement Center, Texas AgriLife Research, and NIEHS P30-ES09106.
Author disclosures: C. A. Warren, K. J. Paulhill, L. A. Davidson, J. R. Lupton, S. S. Taddeo, M. Y. Hong, R. J. Carroll, R. S. Chapkin, and N. D. Turner, no conflicts of interest.
Supplemental Figure 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AC, aberrant crypt; ACF, aberrant crypt foci; AOM, azoxymethane; COX, cyclooxygenase; HMACF, high multiplicity aberrant crypt foci; iNOS, Inducible nitric oxide synthase; PI 3-kinase, phosphatidylinositol 3-kinase.
References
- 1.van Gils CH, Peeters PH, Bueno-de-Mesquita HB, Boshuizen HC, Lahmann PH, Clavel-Chapelon F, Thiebaut A, Kesse E, Sieri S, et al. Consumption of vegetables and fruits and risk of breast cancer. JAMA. 2005;293:183–93. [DOI] [PubMed] [Google Scholar]
- 2.Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–84. [DOI] [PubMed] [Google Scholar]
- 3.van Breda SG, van Agen E, Engels LG, Moonen EJ, Kleinjans JC, van Delft JH. Altered vegetable intake affects pivotal carcinogenesis pathways in colon mucosa from adenoma patients and controls. Carcinogenesis. 2004;25:2207–16. [DOI] [PubMed] [Google Scholar]
- 4.Arikawa AY, Gallaher DD. Cruciferous vegetables reduce morphological markers of colon cancer risk in dimethylhydrazine-treated rats. J Nutr. 2008;138:526–32. [DOI] [PubMed] [Google Scholar]
- 5.Koushik A, Hunter DJ, Spiegelman D, Beeson WL, van den Brandt PA, Buring JE, Calle EE, Cho E, Fraser GE, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst. 2007;99:1471–83. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr. 2007;137:S2576–9. [DOI] [PubMed] [Google Scholar]
- 7.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–39. [DOI] [PubMed] [Google Scholar]
- 8.Hong MY, Chapkin RS, Morris JS, Wang N, Carroll RJ, Turner ND, Chang WC, Davidson LA, Lupton JR. Anatomical site-specific response to DNA damage is related to later tumor development in the rat azoxymethane colon carcinogenesis model. Carcinogenesis. 2001;22:1831–5. [DOI] [PubMed] [Google Scholar]
- 9.Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–30. [DOI] [PubMed] [Google Scholar]
- 10.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin M. NF-κB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–61. [DOI] [PubMed] [Google Scholar]
- 12.Watson WH, Cai J, Jones DP. Diet and apoptosis. Annu Rev Nutr. 2000;20:485–505. [DOI] [PubMed] [Google Scholar]
- 13.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. [DOI] [PubMed] [Google Scholar]
- 14.Dihal AA, de Boer VC, van der Woude H, Tilburgs C, Bruijntjes JP, Alink GM, Rietjens IM, Woutersen RA, Stierum RH. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in F344 rats. J Nutr. 2006;136:2862–7. [DOI] [PubMed] [Google Scholar]
- 15.Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A. Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha-RAS-transformed human colon cells. Carcinogenesis. 2007;28:1021–31. [DOI] [PubMed] [Google Scholar]
- 16.Tanigawa S, Fujii M, Hou DX. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem. 2008;72:797–804. [DOI] [PubMed] [Google Scholar]
- 17.Johnson IT. Anticarcinogenic effects of diet-related apoptosis in the colorectal mucosa. Food Chem Toxicol. 2002;40:1171–8. [DOI] [PubMed] [Google Scholar]
- 18.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signaling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–98. [DOI] [PubMed] [Google Scholar]
- 19.Roymans D, Slegers H. Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem. 2001;268:487–98. [DOI] [PubMed] [Google Scholar]
- 20.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Carrera AC. New functions for PI3K in the control of cell division. Cell Cycle. 2007;6:1696–8. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Plummer SJ, Thompson CL, Tucker TC, Casey G. Association between phosphatidylinositol 3-kinase regulatory subunit p85alpha Met326lle genetic polymorphism and colon cancer risk. Clin Cancer Res. 2008;14:633–7. [DOI] [PubMed] [Google Scholar]
- 23.Phillips WA, St. Clair F, Munday AD, Thomas RJ, Mitchell CA. Increased levels of phosphatidylinositol 3-kinase activity in colorectal tumors. Cancer. 1998;83:41–7. [DOI] [PubMed] [Google Scholar]
- 24.Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. The antiproliferative effect of quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26:1177–81. [PubMed] [Google Scholar]
- 25.Kim WK, Bang MH, Kim ES, Kang NE, Jung KC, Cho HJ, Park JH. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J Nutr Biochem. 2005;16:155–62. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2004;25:647–59. [DOI] [PubMed] [Google Scholar]
- 27.Takeda H, Sonoshita M, Oshima H, Sugihara K-I, Chulada PC, Langenbach R, Oshima M, Taketo MM. Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal polyps. Cancer Res. 2003;63:4872–7. [PubMed] [Google Scholar]
- 28.Ohta T, Takahashi M, Ochiai A. Increased protein expression of both inducible nitric oxide synthase and cyclooxygenase-2 in human colon cancers. Cancer Lett. 2006;239:246–53. [DOI] [PubMed] [Google Scholar]
- 29.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–72. [DOI] [PubMed] [Google Scholar]
- 30.Corona G, Deiana M, Incani A, Vauzour D, Dessi MA, Spencer JPE. Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem Biophys Res Commun. 2007;362:606–11. [DOI] [PubMed] [Google Scholar]
- 31.Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis. 2006;27:1257–65. [DOI] [PubMed] [Google Scholar]
- 32.Patil BS, Pike LM, Yoo KS. Variation in the quercetin content in different colored onions (Allium Cepa L). J Am Soc Hortic Sci. 1995;120:909–13. [Google Scholar]
- 33.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–51. [DOI] [PubMed] [Google Scholar]
- 34.Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, Dougherty ER, Wang N, Lupton JR, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukitsu T, Takayama T, Miyanishi K, Nobuoka A, Katsuki S, Sato Y, Takimoto R, Matsunaga T, Kato J, et al. Aberrant crypt foci as precursors of the dysplasia-carcinoma sequence in patients with ulcerative colitis. Clin Cancer Res. 2008;14:48–54. [DOI] [PubMed] [Google Scholar]
- 36.Pretlow TP, O'Riordan MA, Somich GA, Amini SB, Pretlow TG. Aberrant crypts correlate with tumor incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis. 1992;13:1509–12. [DOI] [PubMed] [Google Scholar]
- 37.Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1271–9. [PubMed] [Google Scholar]
- 38.Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93:525–33. [DOI] [PubMed] [Google Scholar]
- 39.Spencer JP, Rice-Evans C, Williams RJ. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem. 2003;278:34783–93. [DOI] [PubMed] [Google Scholar]
- 40.Duraj J, Zazrivcova K, Bodo J, Sulikova M, Sedlak J. Flavonoid quercetin, but not apigenin or luteolin, induced apoptosis in human myeloid leukemia cells and their resistant variants. Neoplasma. 2005;52:273–9. [PubMed] [Google Scholar]
- 41.Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC. AKT pro-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–5. [DOI] [PubMed] [Google Scholar]
- 42.Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.