Table 1.
Synthesis of Tetrahydropyridines 3 From Ethyl 2-Methyl-2,3-butadienoate and N-Tosylaldiminesa
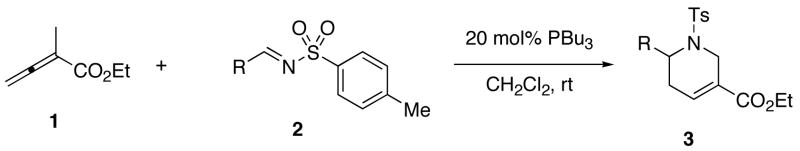 | |||
|---|---|---|---|
| entry | R | product | yield (%)b |
| 1 | Ph (2) | 3 | 94c |
| 2 | 4-MeOC6H4 (2a) | 3a | 99 |
| 3 | 4-MeC6H4 (2b) | 3b | 95 |
| 4 | 3-ClC6H4 (2c) | 3c | 96 |
| 5 | 2-ClC6H4 (2d) | 3d | 93 |
| 6 | 4-FC6H4 (2e) | 3e | 95 |
| 7 | 4-NCC6H4 (2f) | 3f | 98 |
| 8 | 2-F3CC6H4 (2g) | 3g | 98 |
| 9 | 4-O2NC6H4 (2h) | 3h | 86 |
| 10 | 2-HOC6H4 (2m) | 3i | 0 |
| 11 | 2-TBSOC6H4 (2n) | 3j | 93 |
| 12 | 2-pyrrolyl (2l) | 3k | 0 |
| 13 | N-Boc-2-pyrrolyl (2m) | 3l | 99 |
| 14 | 1-naphthyl (2i) | 3m | 96 |
| 15 | 2-furyl (2j) | 3n | 97 |
| 16 | 4-pyridyl (2k) | 3o | 92d |
| 17 | tert-butyl (2o) | 3p | 86e |
| 18 | n-propyl (2p) | 3q | 0f |
1.0 mmol scale.
Isolated yields.
30 mmol scale.
30 mol% PBu3 was used.
3 equiv of Na2CO3 was added.
The imine decomposed to the corresponding aldehyde and p-toulenesulfonamide.
