Table 2.
Synthesis of Tetrahydropyridines 9 From Ethyl 2-Arylmethyl-2,3-butadienaoates and N-Tosylaldiminesa
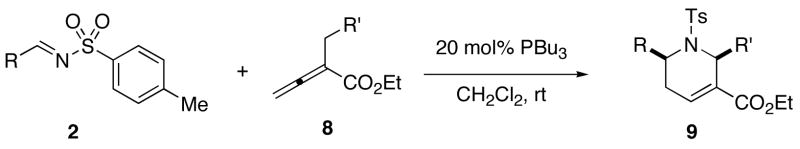 | |||||
|---|---|---|---|---|---|
| entry | R | R ′ | product | yield (%)b | drc |
| 1d | Ph (2) | Ph (8a) | 9a | 92 | 98:2 |
| 2 | Ph (2) | 4-NCC6H4 (8b) | 9b | 99 | 98:2 |
| 3 | Ph (2) | 3-MeOC6H4 (8c) | 9c | 99 | 98:2 |
| 4 | Ph (2) | 2-FC6H4 (8d) | 9d | 99 | 97:3 |
| 5 | Ph (2) | 2-MeC6H4 (8e) | 9e | 82 | 88:12 |
| 6 | 4-MeOC6H4 (2a) | Ph (8a) | 9f | 99 | 97:3 |
| 7 | 4-O2NC6H4 (2h) | Ph (8a) | 9g | 90 | 95:5 |
| 8 | 2-F3CC6H4 (2g) | 4-NCC6H4 (8b) | 9h | 80 | 90:10 |
| 9 | 3-ClC6H4 (2c) | 4-NCC6H4 (8b) | 9i | 99 | 98:2 |
| 10 | 4-MeC6H4 (2b) | 3-MeOC6H4 (8c) | 9j | 99 | 98:2 |
| 11 | 2-ClC6H4 (2d) | 3-MeOC6H4 (8c) | 9k | 96 | 83:17 |
1.0 mmol scale.
Isolated yields.
Diastereoisomeric ratio, determined using 1H NMR spectroscopy.
30 mmol scale.
